Inactivated virus preservation solution and preparation method thereof
A technology for preserving fluids and viruses, applied in biochemical equipment and methods, and microbial measurement/inspection, etc., can solve the problems of increasing the difficulty of washing and removal, guanidine salt residue, secondary transmission of infection, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Preparation of inactivated virus preservation solution: Weigh 59.08 g of guanidine isothiocyanate, 15.76 g of trishydroxymethylaminomethane hydrochloride, 6.74 g of tetrasodium iminodisuccinate, 5 g of sodium dodecylsulfonate, benzene Add 0.5 g of ammonium chloride, 1 g of dithiothreitol, add 800 mL of purified water, adjust the pH value to 6.0 with NaOH, add purified water to make the volume to 1 L, so that the final concentration of guanidine isothiocyanate is 0.1 mol / L, three Hydroxymethylaminomethane hydrochloride is 100mmol / L, tetrasodium iminodisuccinate is 30mmol / L, sodium dodecylsulfonate is 0.50% (w / v) by weight and volume, benzalkonium chloride is The weight to volume ratio is 0.05% (w / v), and the weight to volume ratio of dithiothreitol is 0.10% (w / v). After preparation, the solution was sterilized through a 0.25 μm filter.
[0029] 1. Verification of virus inactivation performance
[0030] In the experimental group, the lentivirus with high-brightness gree...
Embodiment 2
[0032] Example 2 Virus Nucleic Acid Preservation Effect and Nucleic Acid Detection Verification
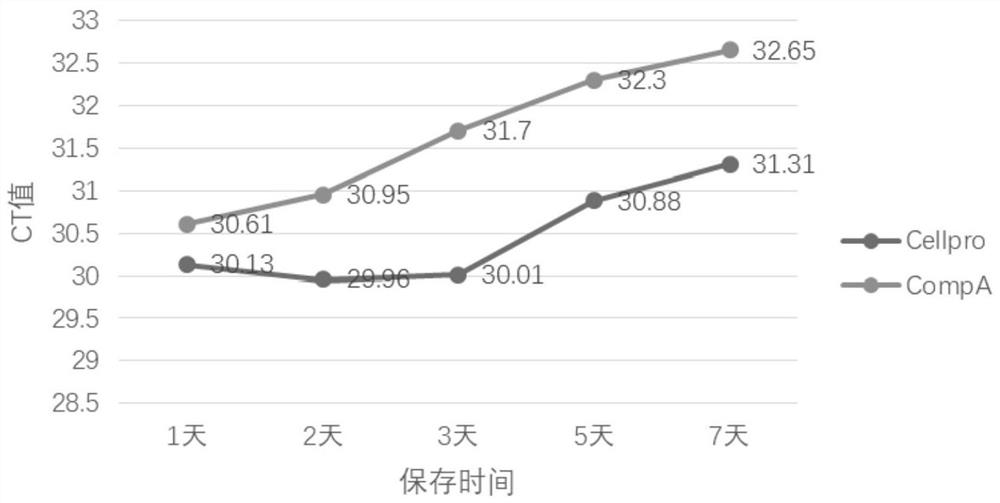
[0033] 1 Sample processing: Get each experimental batch by the virus preservation solution prepared in Example 1, marked as Cellpro 3 tube (2mL / tube) and the virus preservation solution prepared according to the prior art (wherein, the final concentration of sodium citrate The final concentration of proteinase K is 25mmol / L, the final concentration of proteinase K is 200μg / mL, the final concentration of concentrated sulfuric acid is 2.5% (V / V), the final concentration of ethylenediaminetetraacetic acid is 30mmol / L, and the final concentration of ammonium sulfate is 60%. (W / V)) marked as CompA 3 tubes (2mL / tube), add the RNA pseudovirus sample (Goldwise Biotechnology Co., Ltd.) of serial dilution, make the concentration successively 10 3 copies / mL, 10 4 copies / mL, 10 5 copies / mL, store the preservation solution added with pseudovirus samples at room temperature (20-25°C, humidity...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com