Universal virus sample preserving fluid and preparation method thereof
A preservation solution and general-purpose technology, applied in the field of biomedicine, can solve the problems of lack of virus sample preservation solution, etc., to reduce risks, avoid degradation, and ensure accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: the preparation of virus preservation solution
[0042] The first virus preservation solution (referred to as "preservation solution 1"): includes the following components: 100mmol / L of trishydroxymethylaminomethane hydrochloride, 12mmol / L of tetrasodium iminodisuccinate, and 1w / L of sucrose. v%, 0.9w / v% of sodium chloride, 0.2w / v% of sodium lauryl sulfate, 0.8w / v% of Pluracare 1307, 20mmol / L of ethylenediaminetetraacetic acid, 0.8 of casein % w / v, the liquid biological preservative Proclin 300 is 0.2w / v%, the solvent is RNase-free purified water, and the pH of the preservation solution is 8.0.
[0043] The second virus preservation solution (referred to as "preservation solution 2"): includes the following components: 100mmol / L of trishydroxymethylaminomethane hydrochloride, 5mmol / L of tetrasodium iminodisuccinate, and 2w / L of sucrose. v%, sodium chloride 1.5w / v%, sodium dodecyl sulfate 0.2w / v%, Pluracare 1307 1w / v%, ethylenediaminetetraacetic acid 30mmo...
Embodiment 2
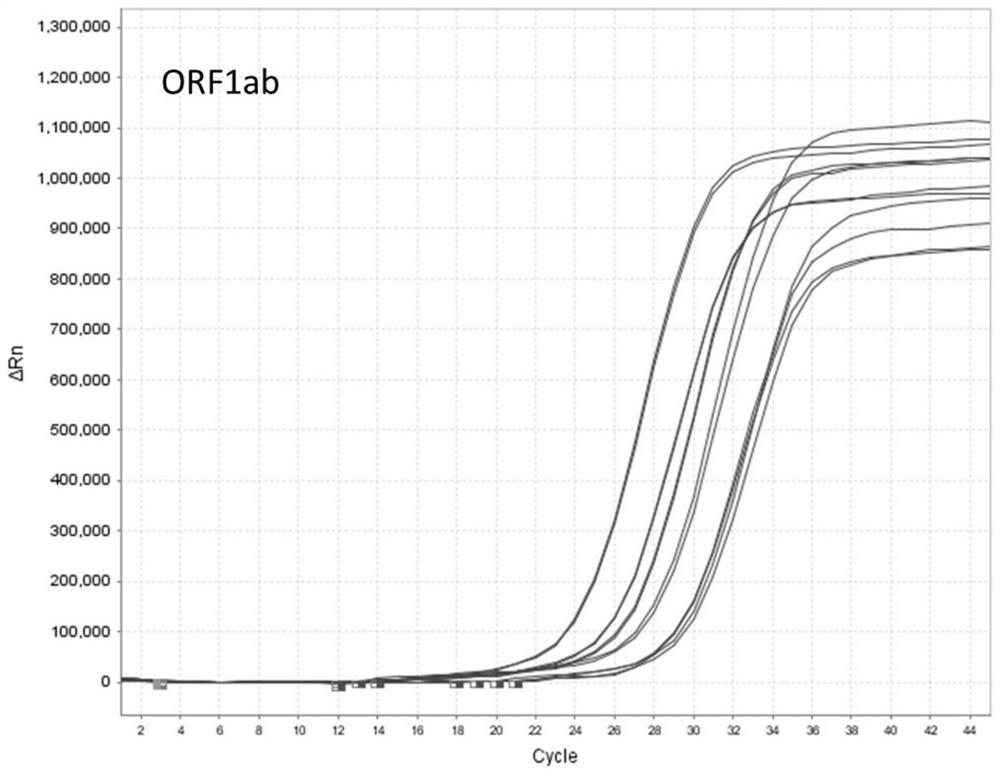
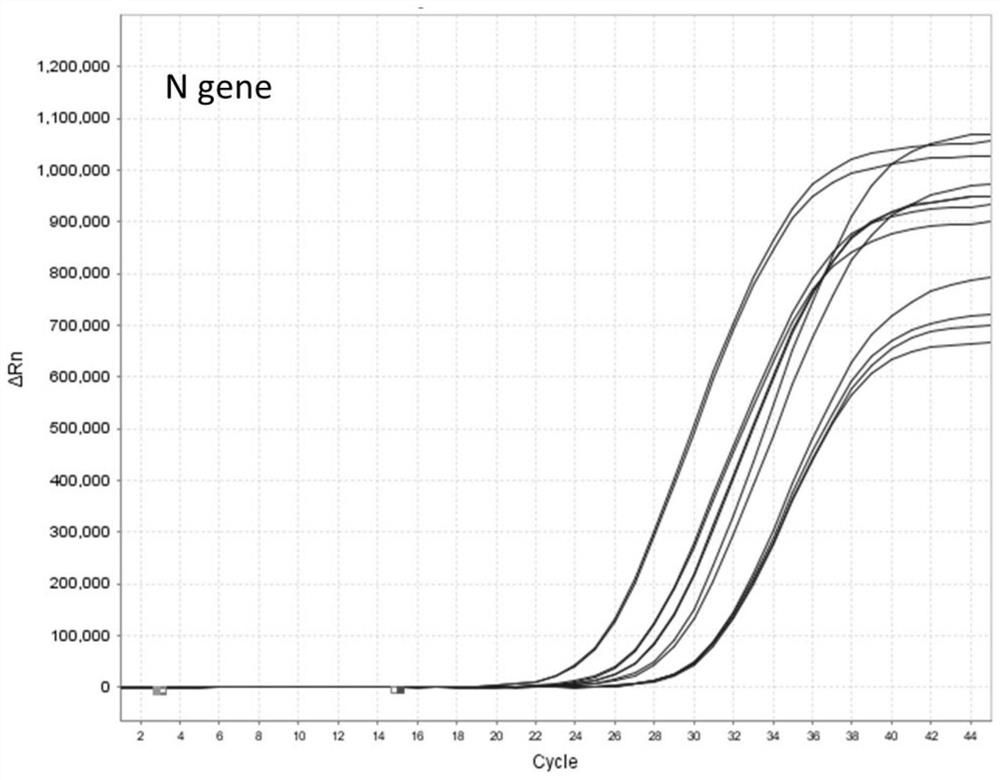
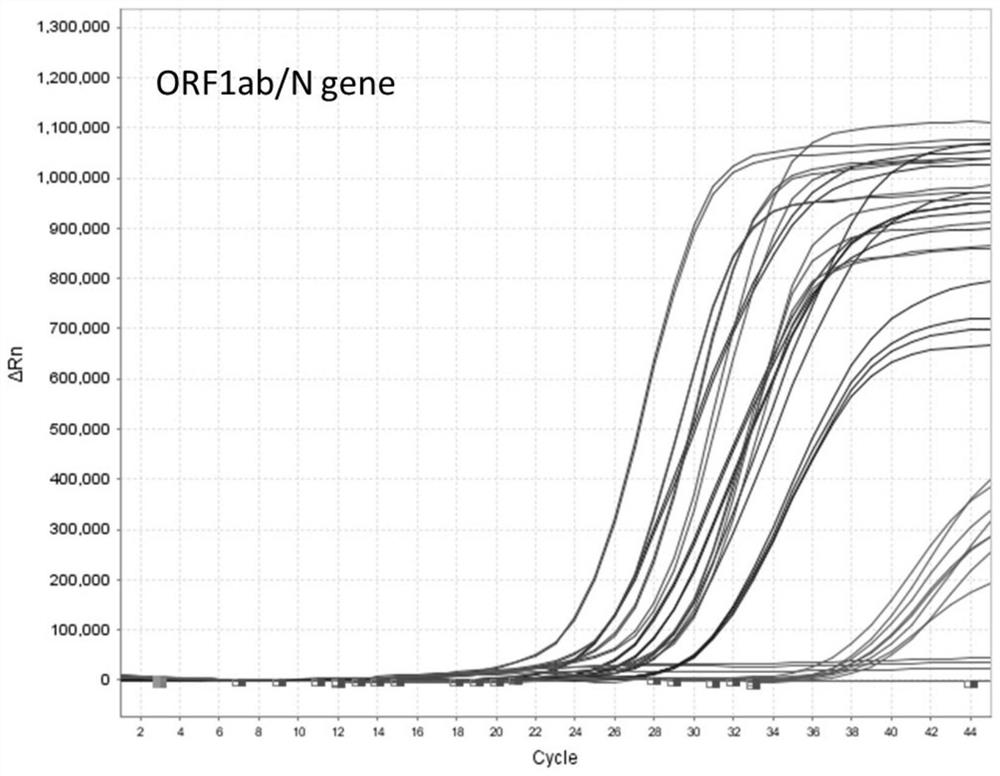
[0046] Example 2: Nucleic acid detection and verification of nucleic acid preservation effect in virus preservation solution
[0047] 1) Add the inactivated virus culture of the new coronavirus (2019-nCOV) to the three kinds of sample preservation solutions in Example 1, and dilute it to the following concentration gradient: 800 TCID 50 、400TCID 50 、200TCID 50 、100TCID 50 、50TCID 50 、25TCID 50 . The Ct value corresponding to each concentration was obtained by amplifying with an approved and listed nucleic acid detection kit (PCR fluorescence method).
[0048] 2) Extraction of viral RNA: Take 200 μL of each sample with the above concentration gradient, and perform nucleic acid extraction according to the instructions of the viral nucleic acid extraction kit (Nanjing Novizan Biotechnology Co., Ltd., Cat.RC311-C1). The final viral RNA purification product elution volume was 50 μL.
[0049] 3) RT-qPCR fluorescence quantitative detection: configure the PCR reaction system ac...
Embodiment 3
[0060] Example 3: Colloidal gold detection and verification of virus preservation solution virus protein preservation effect
[0061] 1) The virus culture with a concentration gradient prepared by diluting the same batch of virus preservation solution in Example 2 above was tested using the protocol of the colloidal gold detection of the new crown antigen (Nanjing Novizan Medical Technology Co., Ltd., Cat.C8602CA). Observe the test card 10 minutes after the start of the test, and judge the result. After 15 minutes, the observation result is invalid.
[0062] 2) The test results shall be judged as follows:
[0063] Negative result: only a red quality control line (C line) can be seen by naked eye.
[0064] Positive result: Two clear red lines can be seen by naked eyes, one is the quality control line (C line) and the other is the T test line.
[0065] Invalid result: no red line visible to the naked eye or only T test line but no quality control line (C line), indicating that...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com