Preparation method of cimetidine
A technology for cimetidine and imidazole, applied in the field of preparation of cimetidine, can solve the problems of difficulty in obtaining imidazol-4-yl) acetate, difficulty in large-scale production, difficulty in large-scale production and the like, and avoid odor Problems, high productivity, environmental friendliness and improved safety effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
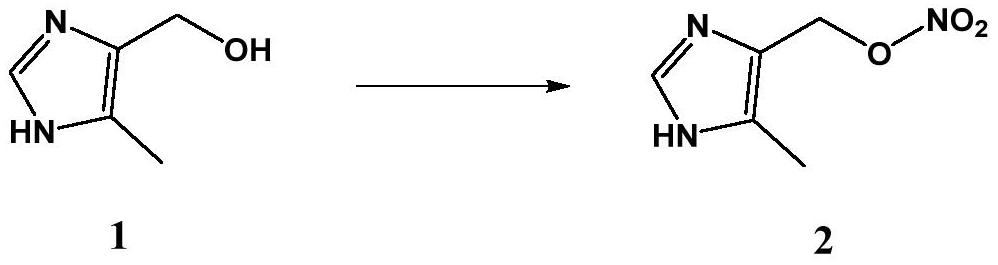
[0034] Add (5-methyl-1H-imidazol-4-yl)methanol (1.12g, 10.0mmol), 50mL of methanol, and 10mL of acetic anhydride into a 100mL three-necked flask, stir and mix; cool down to 10°C, at this temperature Sodium nitrite (0.76 g, 11.0 mmol) was added in batches, the temperature was naturally raised to room temperature and stirring was continued. The reaction progress was monitored by TLC until the raw material (5-methyl-1H-imidazol-4-yl)methanol completely disappeared, and then the reaction was continued for 0.5h, and the total reaction time was about 3h. After the reaction was finished, the reaction solution was naturally cooled to room temperature, then it was poured into 100 mL of water, the precipitate was collected, recrystallized from ethanol to obtain (5-methyl-1H-imidazol-4-yl) methyl nitrate 1.44 g , yield 92.0%. ESI-MS: m / z 158 [M+H]+.
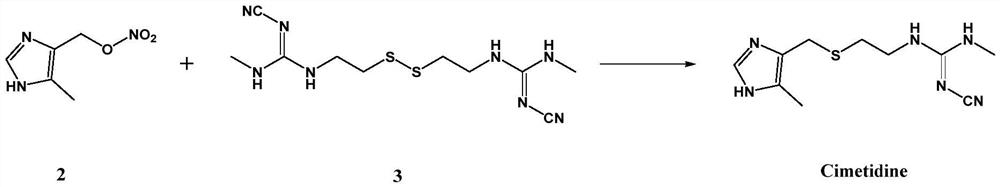
[0035] Under nitrogen protection, (5-methyl-1H-imidazol-4-yl)methyl nitrate (1.26g, 8.0mmol), absolute ethanol (75mL), triethylamine (0....
Embodiment 2
[0037](5-Methyl-1H-imidazol-4-yl)methyl nitrate was synthesized in the same manner as in Example 1. Then, under nitrogen protection, (5-methyl-1H-imidazol-4-yl)methyl nitrate (1.26g, 8.0mmol), anhydrous methanol (80mL), pyridine (0.5mL ), stirred to dissolve, then added cobalt chloride (0.152 g, 1.2 mmol) and continued stirring for 15 min. Subsequently, the ether of N-cyano-N'-methyl-N"-mercaptoethylguanidine (i.e. 1,1'-(dithiodiylbis(ethane-2,1-diyl))bis( 2-cyano-3-methylguanidine)) (1.50g, 4.8mmol) was dissolved in anhydrous methanol (30mL) and slowly added dropwise, stirring was continued during the dropwise addition, and stirring was continued at room temperature after the dropwise addition was completed. .TLC monitors the reaction process, disappears completely to the point of raw material (5-methyl-1H-imidazol-4-yl) methyl nitrate, then continues to react for 1h, and the total reaction time is about 6h. After the reaction finishes, make the reaction solution Naturally ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com