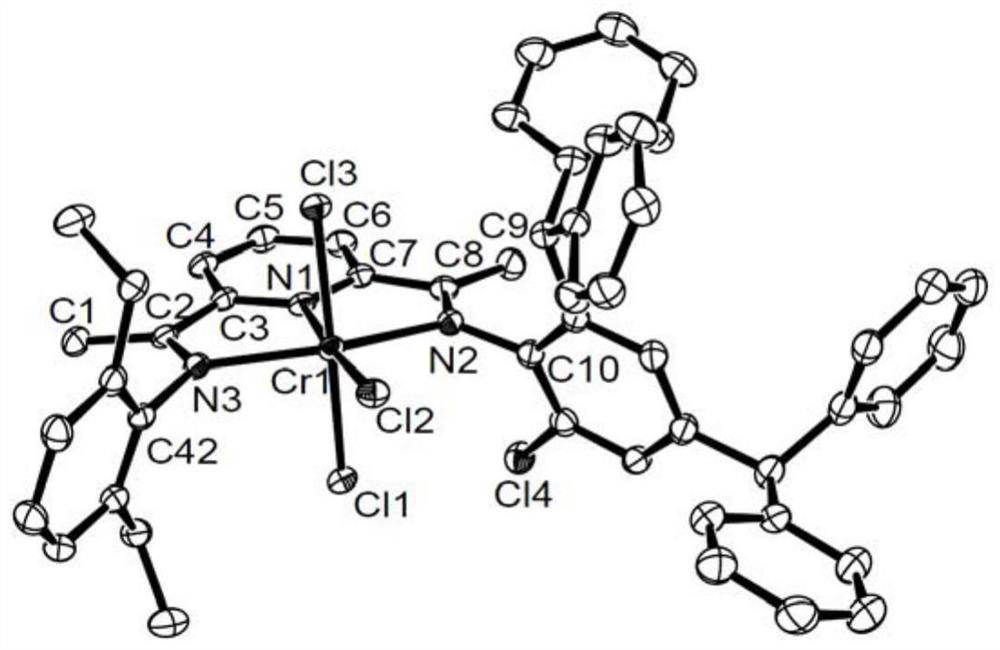
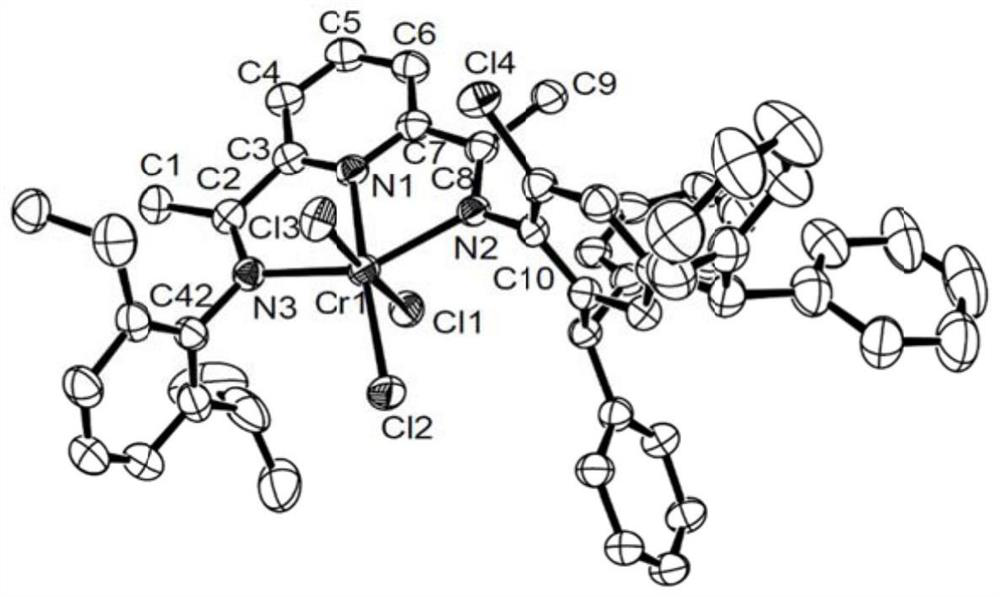
Chromium pyridinediimide complexes, methods for their preparation and use for the preparation of polyolefins
A technology of complexes and ligand compounds, applied in the field of polyolefin catalysts, can solve the problems of poor thermal stability of late transition metal complexes, reduced catalyst activity, complex catalytic products and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] Example 1. Preparation of Compound IA (2-acetyl-6-(1-(2,4-bis(benzhydryl)-6-chloro-anilino) ethyl)pyridine)
[0092] Weigh 4.00g (24.5mmol) of 2,6-diacetylpyridine, 11.27g (24.5mmol) of 2,4-bis(benzhydryl)-6-chloro-aniline and add it to the reaction flask, Add about 150 mL of toluene, and then add a catalytic amount (20%) of p-toluenesulfonic acid in the reaction system. After 10 h of stirring at reflux temperature, the reaction mixture was filtered under heating, and all volatiles were evaporated under reduced pressure. Then, the obtained crude product was subjected to column chromatography through a basic alumina column, and was eluted with a mixed solvent of petroleum ether and ethyl acetate as an eluent (25 / 1), and the solvent was removed to obtain 3.39 g of a light yellow powder , namely compound IA, yield: 22%.
[0093]
[0094] The structural confirmation data are as follows:
[0095] FTIR (cm -1 ):3025(w), 2928(m), 1699(ν(C=O), s), 1658(ν(C=N), s), 1581(m...
Embodiment 2
[0099] Example 2. Ligand L1 (2-(1-(2,4-bis(benzhydryl)-6-chloro-anilino)ethyl)-6-(1-(2,6-dimethyl - the preparation of anilino) ethyl) pyridine)
[0100] Weigh 2.00g (3.19mmol) of compound IA and catalytic equivalent p-toluenesulfonic acid (0.638mmol) into 40mL of toluene solvent, heat and stir to reflux, then add 0.42g (3.50mmol) 2,6-dimethyl Aniline was transferred to the reaction flask, and the reaction mixture was heated to reflux for 10h. Cool to room temperature and evaporate volatiles in vacuo. Then, the obtained unprocessed residual solid was subjected to column chromatography (using a mixed solvent of petroleum ether and ethyl acetate 200:1 (v / v) as eluent) to elute through a basic alumina column, and the solvent was removed. 0.23 g of light yellow powder was obtained, namely ligand L1, yield: 20%.
[0101]
[0102] The structural confirmation data are as follows:
[0103] FTIR (cm -1 ):3028(w), 2937(w), 1643(ν(C=N), s), 1569(w), 1496(w), 1453(s), 1365(s), 132...
Embodiment 3
[0106] Example 3. Ligand L2 (2-(1-(2,4-bis(benzhydryl)-6-chloro-anilino)ethyl)-6-(1-(2,6-diethyl) - the preparation of anilino) ethyl) pyridine)
[0107] Weigh 2.00g (3.19mmol) of compound IA and catalytic equivalent p-toluenesulfonic acid (20%) into 40mL of toluene solvent, heat and stir to reflux, then add 0.52g (3.50mmol) 2,6-diethyl Aniline was transferred to the reaction flask, and the reaction mixture was heated to reflux for 10h. Cool to room temperature and evaporate volatiles in vacuo. Then, the obtained unprocessed residual solid was subjected to column chromatography (using a mixed solvent of petroleum ether and ethyl acetate 200:1 (v / v) as eluent) to elute through a basic alumina column, and the solvent was removed. 0.20 g of light yellow powder was obtained, namely ligand L2, yield: 18%.
[0108]
[0109] The structural confirmation data are as follows:
[0110] FTIR (cm -1 ):3024(w), 2930(w), 1639(ν(C=N), s), 1567(w), 1493(w), 1449(s), 1365(s), 1326(w), 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| dispersity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com