Method for synthesizing polyunsaturated side group-containing polyester or polyether ester by using rare earth catalyst and post-modification of polyunsaturated side group-containing polyester or polyether ester
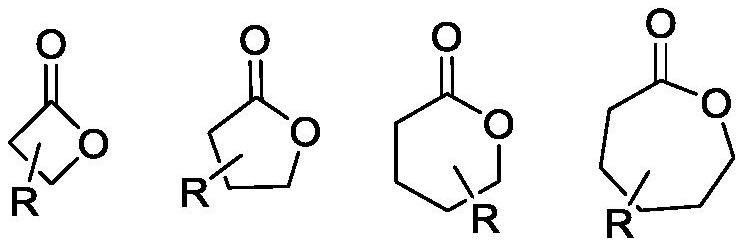
A technology of rare earth catalyst and side-group polyester, which is applied in the field of synthesis of cyclic lactone and oxetane, polyester or polyether ester, can solve the problem that EVL cannot carry out ring-opening polymerization, etc., and achieves a simple and efficient preparation method. The effect of cheap and easy-to-obtain raw materials and high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
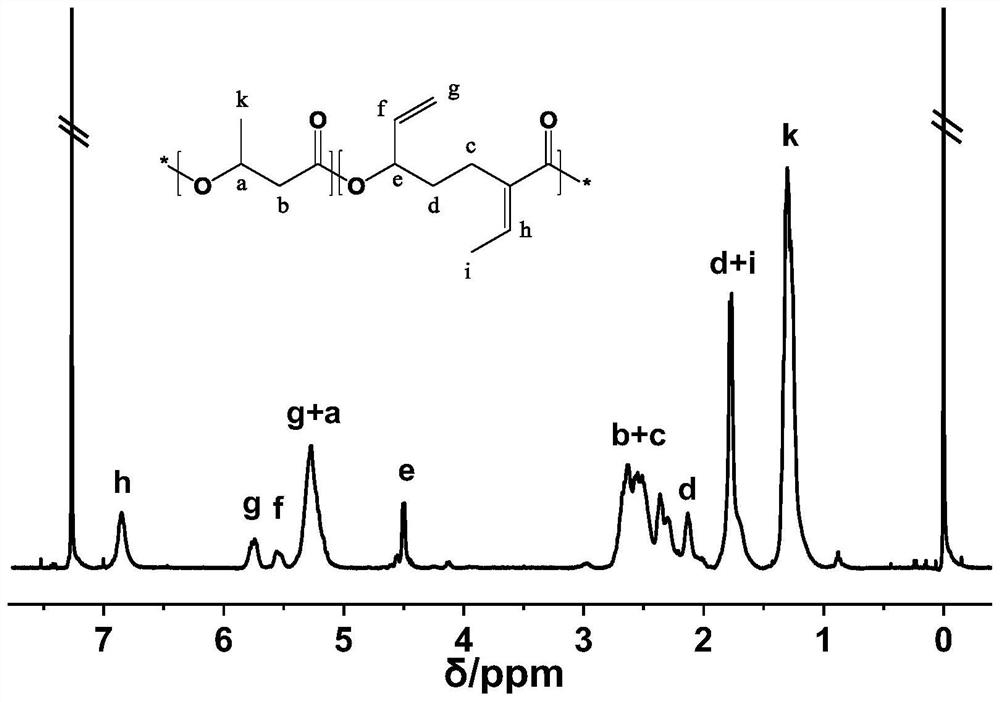
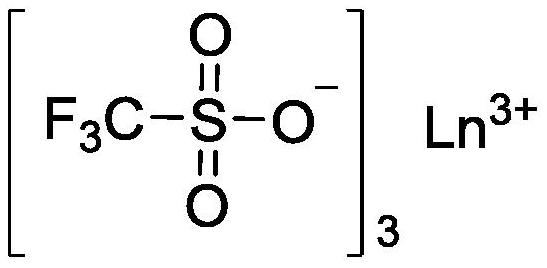
[0053] Add 0.014g (0.028mmol) of Sc(OTf) to the flame-dried reaction flask 3 , then add 0.170g (1.118mmol) of EVL and 0.142g (1.654mmol) of β-butyrolactone (β-BL), shake evenly, and dissolve the catalyst in the monomer. Sc(OTf) 3 The molar ratio to the monomer is 1:100. After sealing, it was placed in an oil bath at 70°C for 2 hours to react. After the reaction, the mixture was poured into n-hexane to precipitate and filtered, and the obtained polymer was vacuum-dried for 2 days to obtain polyester with a yield of 90%. The SEC weight average molecular weight of the obtained polymer was 2.9 kDa, and the molecular weight distribution was 2.87.
[0054] The resulting product was dissolved in deuterated chloroform, and carried out 1 H NMR test, the obtained product nuclear magnetic proton spectrogram sees attached figure 1 . figure 1 It can be seen in the figure that at the low field of the H NMR spectrum, the hydrogen atom signals h, g, and f on the unsaturated bonds in the ...
Embodiment 2
[0056] Other polymerization conditions are identical with embodiment 1, difference is to use Lu (OTf) 3 as a catalyst. Monomer and Lu(OTf) 3 The molar ratio was 200:1, and the reaction was carried out in an oil bath at 50° C. for 24 hours, and the yield of the obtained polyester was 93%. The SEC weight average molecular weight of the obtained polymer was 3.9 kDa, and the molecular weight distribution was 2.45.
Embodiment 3
[0058] Other polymerization conditions are identical with embodiment 1, difference is to use La(OTf) 3 as a catalyst. Monomer and La(OTf) 3 The molar ratio was 300:1, and the reaction was carried out in a thermostat at 25° C. for 7 days, and the yield of the obtained polyester was 95%. The SEC weight average molecular weight of the obtained polymer was 5.9 kDa, and the molecular weight distribution was 1.89.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com