Aldehyde ketone reductase KmAKR mutant and application thereof in catalytic synthesis of chiral alcohol
A reductase and mutant technology, applied in the field of aldehyde-ketone reductase KmAKR mutants, can solve the problems of large amount of catalyst, low asymmetric reduction activity, low activity of non-natural ketoester substrates, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: Construction and screening of aldehyde and ketone reductase mutant library
[0034] 1. Starting strain:
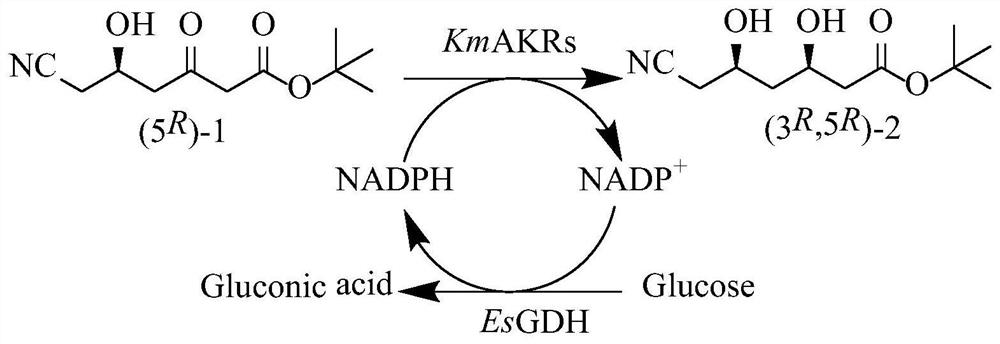
[0035] Taking the engineering bacteria E.coli BL21(DE3) / pET28a(+)-kmakr-W297H / Y296W / K29H / Y28A / T63M in the patent application CN201910932502.0 as the starting strain, denoted as strain M5, activated and extracted the plasmid pET28a(+) -kmakr-W297H / Y296W / K29H / Y28A / T63M, wherein the amino acid sequence of aldehyde and ketone reductase kmakr-W297H / Y296W / K29H / Y28A / T63M is shown in SEQ ID NO.1, and the coding gene sequence is shown in SEQ ID NO.2 Show.
[0036] 2. Single mutation:
[0037] (1) Construction of mutant library
[0038] The preparation of the aldehyde and ketone reductase KmAKR mutant library was achieved by site-directed mutagenesis and random mutagenesis. The vector pET28a(+)-kmakr-W297H / Y296W / K29H / Y28A / T63M in the strain M5 was used as a template and the primers in Table 1 were used for polymerization. Enzyme chain reaction (PCR). Transfer ...
Embodiment 2
[0074] Embodiment 2: Induced expression of starting strain and mutant strain aldehyde ketone reductase and glucose dehydrogenase
[0075] 1. Glucose dehydrogenase genetically engineered bacteria: Insert the glucose dehydrogenase gene esgdh (GenBank No.KM817194.1, nucleotide sequence shown in SEQ ID NO.7) from Exiguobacterium sibirium DSM 17290 into pET28b (+) between the Nco I and Xho I restriction sites, construct a recombinant expression vector; and transfer this expression vector into E.coli BL21(DE3), pick a single colony and inoculate it into LB medium, at 37°C After culturing for 12 hours, it was confirmed by sequencing that the glucose dehydrogenase was successfully constructed, and E. coli BL21(DE3) / pET28b(+)-esgdh was obtained.
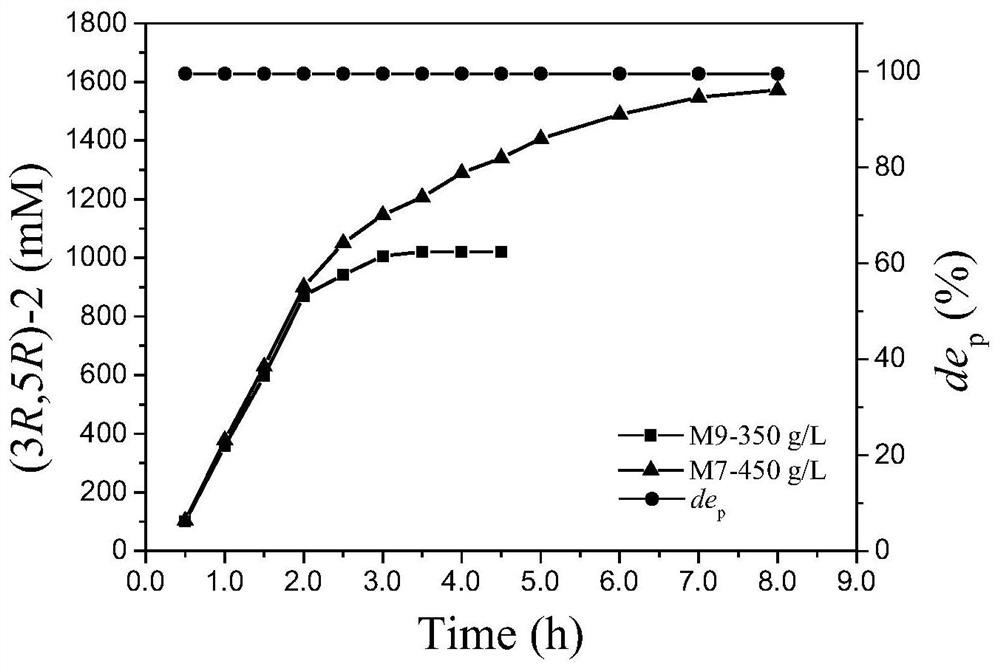
[0076] 2. Induced expression: Inoculate the starting strain M5, mutant strain M7, mutant strain M9, and E.coli BL21(DE3) / pET28b(+)-esgdh in Example 1 into 10 mL of kanamycin containing a final concentration of 50 μg / mL, respectively. In LB liq...
Embodiment 3
[0084] Example 3: Purification of aldehyde and ketone reductase in the starting strain of aldehyde and ketone reductase and its mutant strains
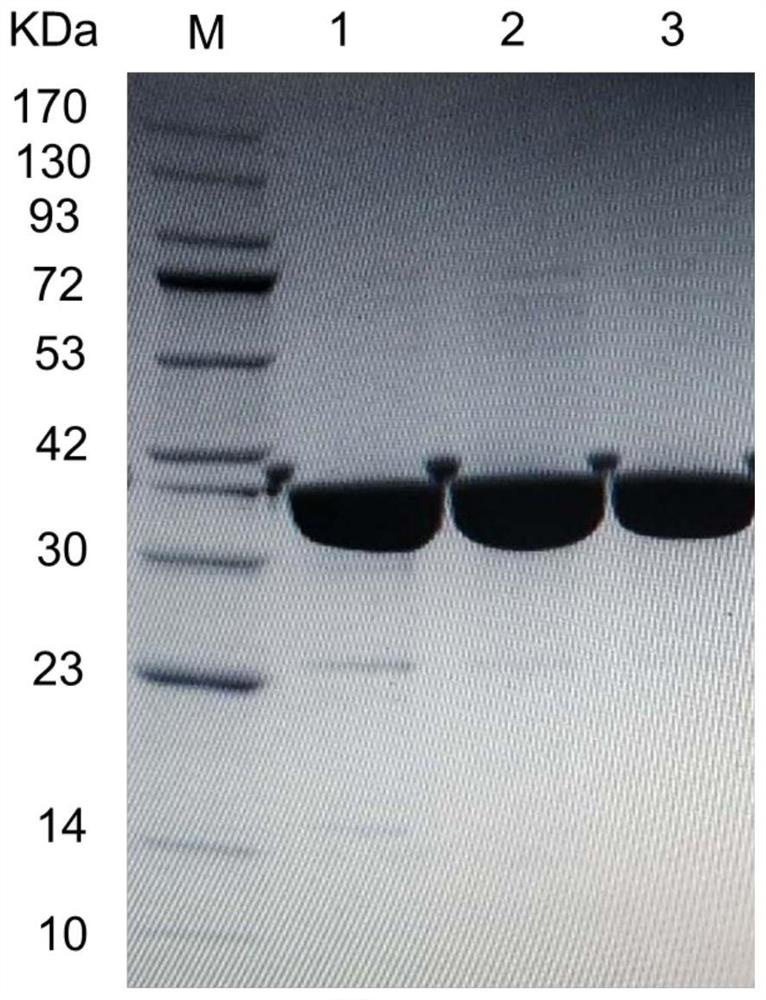
[0085] The starting strain M5, the aldehyde and ketone reductase mutant strain M7 obtained in Example 2, and the wet bacterial cell of the mutant strain M9 were washed twice with 0.9 g / mL physiological saline. According to the amount of 100g / L of the total amount of wet bacteria, add pH 7.0, 100mM PBS buffer to resuspend, and ultrasonically break on the ice-water mixture for 6min. Enzyme solution. By centrifuging at 8000rpm at 4°C for 10min, 20mL of the supernatant was collected, and after microfiltration through a 0.45μm membrane, the filtrate was purified with a Ni affinity column to purify the mutant protein.
[0086] The mutant protein was purified using a nickel affinity column (1.6×10 cm, Bio-Rad, USA), and the specific operation was as follows: ① Pre-equilibrated with buffer A (pH 7.0 containing 300 mM NaCl, 20 mM imidazole, 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com