Three-way catalyst system and application thereof in ethylene oligomerization reaction
A three-way catalyst and catalyst technology, applied in catalytic reactions, catalysts, carbon compound catalysts, etc., can solve the problems of adsorbing catalyst components, affecting air tightness, affecting mass transfer, etc., to reduce the generation of high polymers, improve Wall-hanging slurry, the effect of improving selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
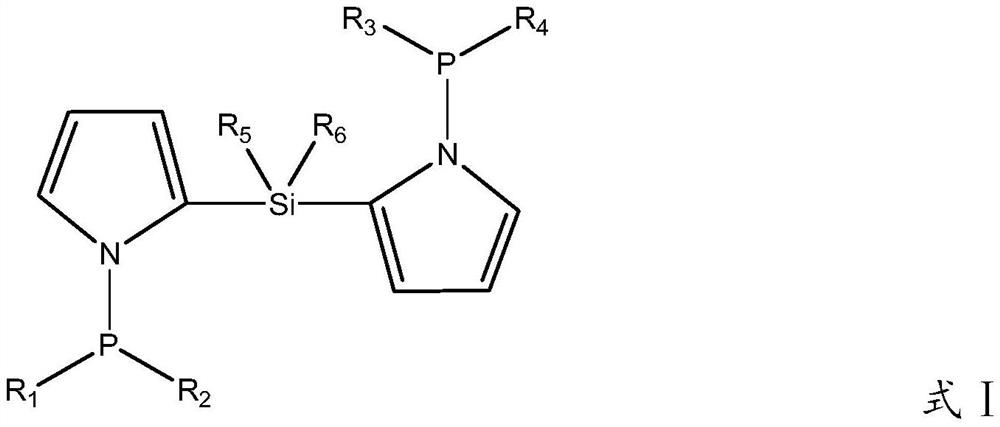
[0061] The preparation method of phosphine-nitrogen ligand (L1):
[0062] Pyrrole (2.25mol) was dissolved in 180ml (2.80mol) of dichloromethane solution to obtain a reaction solution;
[0063] At -78°C, 1.5 mol of n-butyllithium was added dropwise to the reaction solution under stirring, and the compound (dichlorodimethylsilane 1 mol) and 8 mL (0.11 mol) of trifluoroacetic acid were continuously added to it. , slowly warming up to room temperature and reacting for 50h to obtain reaction solution 1;
[0064] The reaction liquid 1 was filtered, and the compound represented by the structure of formula III (1.25 mol of diphenylphosphine chloride) was added to the obtained filtrate at -78°C, and the reaction liquid was slowly raised to room temperature to obtain the reaction liquid 2;
[0065] The reaction solution 2 was purified by column chromatography (washed with tetrahydrofuran, and the aspect ratio was 2), and then recrystallized at 80° C. to obtain the ligand (L1).
[0066...
Embodiment 2
[0069] The difference between the preparation method of the phosphine-nitrogen ligand (L2) and the phosphine-nitrogen ligand (L1) in Example 1 is that the compound represented by the structure of formula III added is 2-fluorodiphenylphosphine chloride.
[0070]
[0071] The NMR data of the above-mentioned ligand (L2) are as follows: 1H NMR (400MHz, CDCl3): 7.24-7.44 (m, 20H), 6.25-6.34 (m, 4H), 0.14 (s, 6H).
Embodiment 3
[0073] The difference between the preparation method of the phosphine-nitrogen ligand (L3) and the phosphine-nitrogen ligand (L1) in Example 1 is that the compound represented by the structure of formula III added is 3-fluorodiphenylphosphine chloride.
[0074]
[0075] The NMR data of the above-mentioned ligand (L3) are as follows: 1H NMR (400MHz, CDCl3): 7.24-7.44 (m, 20H), 6.25-6.34 (m, 4H), 0.14 (s, 6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com