Method for preparing alkane carboxylic acid by increasing alkane carbon chains
An alkane carboxylic acid and carbon chain technology, applied in the field of alkane carboxylic acid synthesis, can solve problems such as being unsuitable for industrial production and harsh operation requirements.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1 8-Methyl nonanoic acid synthesis operation
[0046] 8-Methylnonanoic acid
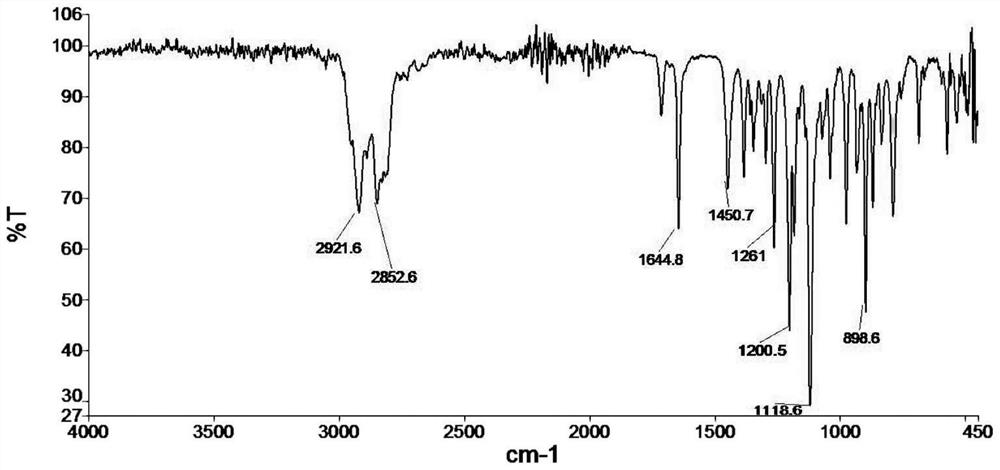
[0047] Add 78.5g cyclohexanone, 83.6g morpholine, catalyzer p-toluenesulfonic acid (TsOH) 1.0g, 200mL entrainer toluene in 500mL reaction flask, stir, heat and reflux to separate water to the calculated amount (14.4g), Then vacuum distillation reclaims toluene 200mL, obtains 170g 1-morpholine-1-cyclohexene crude product, and its infrared spectrogram is as follows figure 1 shown.
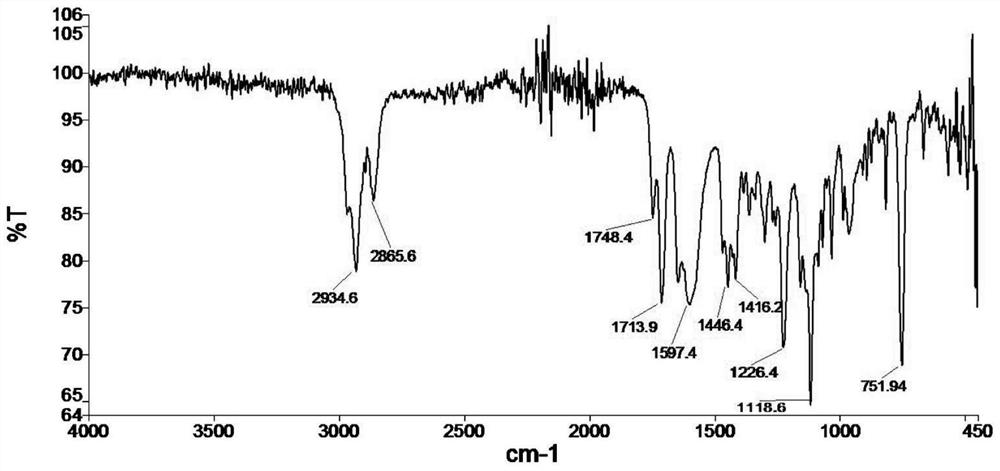
[0048]To the obtained 100.0g crude 1-morpholine-1-cyclohexene, add 300.0mL of chloroform to dilute, then add 121.7g of triethylamine, after cooling down to below 10°C, slowly drop in 127.3g of isobutyryl chloride, dropwise After that, the stirring reaction was continued for 3 hours. Then add 110.0mL of 30% w / w hydrochloric acid aqueous solution, heat and reflux for 2 hours and separate the liquid, separate the chloroform layer and the water layer, and separate the water layer after neutralization with p...
Embodiment 2
[0050] Example 2 7-methyl octanoic acid synthesis operation
[0051] 7-methyloctanoic acid
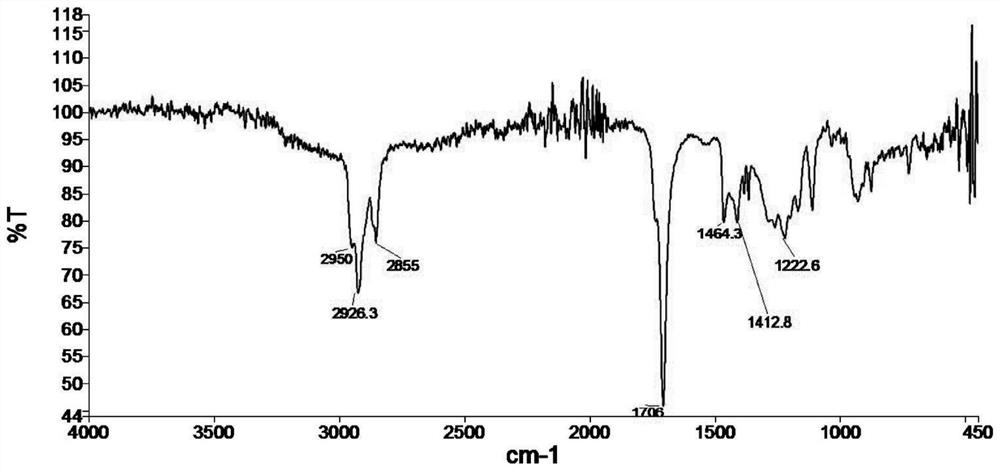
[0052] Add 84.1g of cyclopentanone, 87.1g of morpholine, 1.0g of catalyst p-toluenesulfonic acid, 200mL of entrainer toluene into a 500mL reaction flask, stir, heat and reflux until the water is separated to the calculated amount (18.1g), and then depressurize Recover toluene 200mL by distillation, obtain 163g 1-morpholine-1-cyclopentene crude product, its infrared spectrogram is as follows Figure 4 shown.
[0053] Add 500mL of chloroform to the obtained crude product of 1-morpholine-1-cyclopentene for dilution, then add 210.2g of triethylamine, after cooling down to below 10°C, slowly drop in 220.8g of isobutyryl chloride, and continue stirring React for 3 hours. Then add 110.0mL of 30% w / w hydrochloric acid aqueous solution, heat and reflux for 2 hours and separate the liquid, separate the chloroform layer and the water layer, and separate the water layer after neutralization wit...
Embodiment 3
[0055] Example 3 9-Methyldecanoic Acid Synthesis Operation
[0056] 9-methyldecanoic acid
[0057] According to the method in Example 1, add 300.0mL chloroform to dilute the obtained 100.0g 1-morpholine-1-cyclohexene crude product, add 121.7g triethylamine, after cooling to below 10°C, slowly drop into 144.1g isovaleryl chloride, after dripping, continue to stir and react for 3 hours. Then add 110.0mL of 30% hydrochloric acid aqueous solution, heat and reflux for 2 hours and then separate the layers, separate the chloroform layer and the water layer, and separate the water layer after neutralizing with potassium hydroxide, collect the mixture of triethylamine and morpholine, then dry and Fractional distillation recovers triethylamine and morpholine for later use. The chloroform layer was washed twice with water, dried and filtered over anhydrous sodium sulfate, and concentrated to obtain 65.5g crude product 2-(3-methylbutyryl)cyclohexanone compound, and its infrared spectrum...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com