Preparation method of 3,3',4,4'-tetraaminobiphenyl
A technology of tetraaminobiphenyl and amino, which is applied in the field of compound synthesis, can solve problems such as low yield, long route, and large amount of three wastes, and achieve the effects of short steps, reduced energy consumption, and reduced production volume
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] A preparation method of 3,3',4,4'-tetraaminobiphenyl provided in the embodiment of the present invention comprises the following steps:
[0037] Step S1: In the presence of the first catalyst and the second catalyst, the dichlorobiphenyl diamine is subjected to an amino substitution reaction with an ammoniating reagent to obtain a crude product of 3,3',4,4'-tetraaminobiphenyl, wherein , the first catalyst is a mixture of specified amino acid and cuprous salt, and the second catalyst is a phase transfer catalyst.
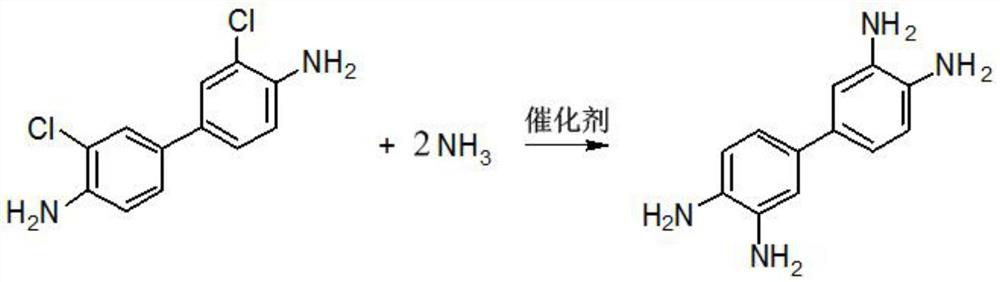
[0038] In step S1, dichlorobiphenyldiamine (also known as 3,3'-dichlorobiphenyl-4,4'-diamine) undergoes an amino substitution reaction with an ammoniating reagent, and the reaction equation is shown in the following formula:
[0039]
[0040] NH in the above formula 3 Provided by ammoniating reagents.
[0041] Step S2: post-processing the crude 3,3',4,4'-tetraaminobiphenyl product to obtain a purified 3,3',4,4'-tetraaminobiphenyl product.
[0042] The pr...
Embodiment 1
[0062] Adding 173.0g (equivalent to 0.53mol) of dichlorobenzidine diamine and 500g of concentration successively in the beating still with ice water cooling is the ammoniacal liquor of 25%, and after beating, it is transferred into 0.4g cuprous chloride, 5.2 In the autoclave of 5.0g proline and 5.2g tetrabutyl ammonium chloride, replace the air in the autoclave with nitrogen four times, then use 248g concentration to wash the beating autoclave after being 25% ammoniacal liquor and change over to the autoclave together. The temperature of the system is controlled to not exceed 10° C. during the whole process of beating, transferring, replacing and washing.
[0063] Turn on the stirring of the autoclave and continue to feed 170g of ammonia into the autoclave. The autoclave was closed and the temperature was raised. After about 1.5 hours, the temperature of the reaction system in the autoclave was raised to 170° C., and the pressure was 2.7 MPa, and the reaction was kept at this...
Embodiment 2
[0067] Add 173.0g (equivalent to 0.53mol) dichlorobenzidine diamine and 500g concentration of ammonia water of 25% successively in the beating still that cools down with ice water, change into 0.2g cuprous chloride, 6.9g cuprous chloride in advance after beating In the autoclave of histidine and 6.9g tetrabutylammonium chloride, the air in the autoclave was replaced with nitrogen four times, and then the beating autoclave was washed with 248g concentration of 25% ammonia water and transferred to the autoclave. The temperature of the system is controlled to not exceed 10° C. during the whole process of beating, transferring, replacing and washing.
[0068] Turn on the stirring of the autoclave and continue to feed 170g of ammonia into the autoclave. The autoclave was closed and the temperature was raised. After about 1.5 hours, the temperature of the reaction system in the autoclave was raised to 170° C., and the pressure was 2.7 MPa, and the reaction was kept at this temperat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com