IR820 and atorvaquone carrier-free self-assembled nanoparticles and preparation method and application thereof
A technology of IR820 and atovaquone, which is applied in the field of medicine, can solve the problems of low bioavailability and easy removal of hydrophilic drug IR820, and achieve good biocompatibility, improved photothermal treatment effect, and uniform particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] In yet another specific embodiment of the present invention, the preparation method of the above-mentioned nanoparticles is provided, the preparation method comprising: dissolving IR820 in water, dissolving atovaquone in an organic solvent, adding the atovaquone organic solvent solution into the IR820 aqueous solution , stirred, dialyzed, passed through a membrane, and obtained.
[0035] Wherein, the organic solvent can be selected from one or more of dimethyl sulfoxide, methanol, ethanol, dichloromethane, acetonitrile, ethyl acetate, etc., and the inventors have found that only dimethyl sulfoxide Sulfone can achieve good solubility of atovaquone, so the solvent is finally preferably dimethyl sulfoxide.
[0036] In the above preparation process, IR820, atovaquone dissolved in a solvent, and the mixing of the two solutions must be carried out under dark conditions.
[0037] In yet another specific embodiment of the present invention, the concentration of IR820 in the IR...
Embodiment 1
[0052] Example 1 Preparation of IR820 / ATO nanoparticles
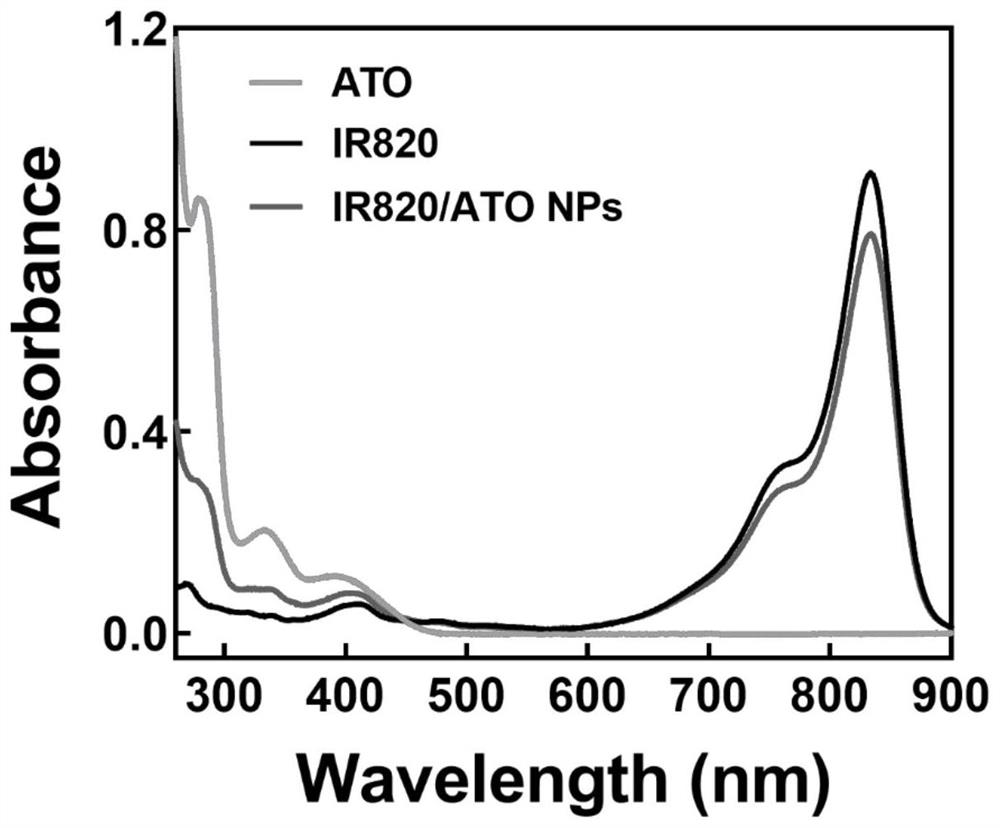
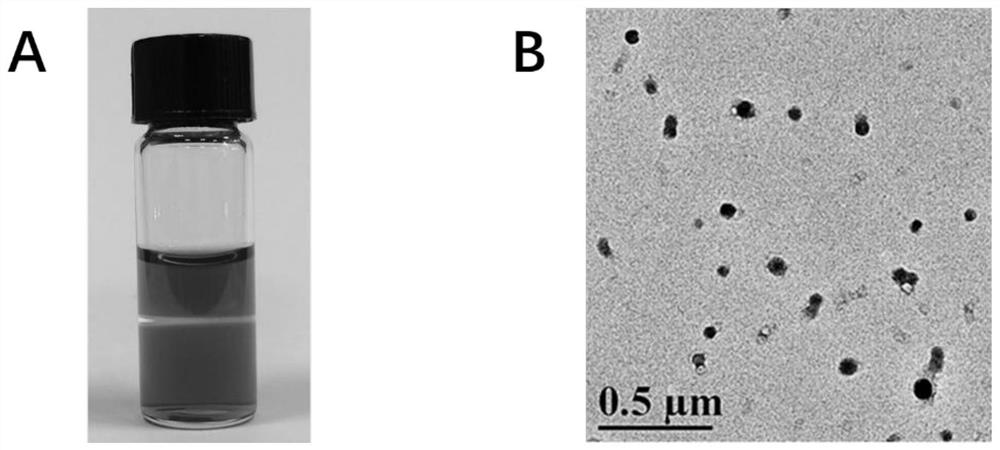
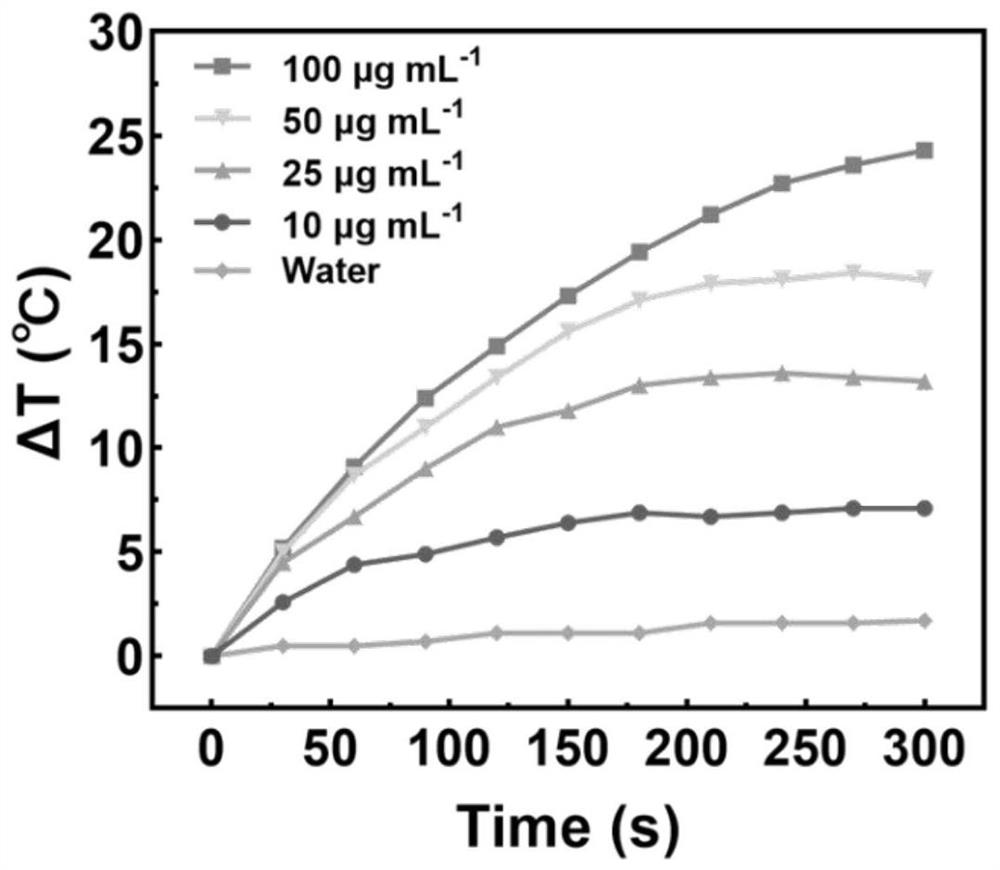
[0053] Precisely weigh a certain amount of atovaquone with an analytical balance and disperse it in dimethyl sulfoxide (DMSO) to make a 10 mg / mL solution, and take a certain amount of IR820 and disperse it in water to make a 0.5 mg / mL solution. Slowly drop the DMSO solution of atovaquone into the aqueous solution of IR820 under rapid stirring conditions, and stir for 20 minutes to obtain a green solution. The mass ratio of atovaquone:IR820 is 2:1. After the reaction, dialyze with distilled water for 3 hours to remove unbound free drug and organic solvent, and pass through a 0.8 μm filter membrane. The UV spectrum of the nanoparticles is as figure 1 As shown, the particle size of nanoparticles is ~110nm, and its appearance and aggregation state are as follows figure 2 shown.
Embodiment 2
[0054] Example 2 Composition ratio of IR820 / ATO nanoparticles detected by ultraviolet spectrophotometry (UV)
[0055] Precisely measure 1 volume of IR820 / ATO nanoparticle solution, add 10 volumes of dimethyl sulfoxide, and sonicate in an ice bath for 5 minutes to fully disintegrate the nanoparticle structure, dilute with distilled water: dimethyl sulfoxide = 1:10 solvent solution, dilute the above solution by an appropriate multiple. Using the solvent of distilled water: dimethyl sulfoxide = 1:10 as a blank reference, the absorbance was obtained by ultraviolet scanning. The results are as follows: figure 1 As shown, by calculating the absorbance values of the diluent at 278nm and 833nm, it can be seen that the mass ratio of IR820:ATO drug in the newly prepared IR820 / ATO nanoparticles is close to 1:2, and the average encapsulation efficiency of the two drugs can reach More than 70%. By UV spectrum ( figure 1 ) can confirm the successful preparation of IR820 / ATO nanoparti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com