New application of sevelamer
A technology of Sevelamer and Sevelamer, which is applied in the field of medicine, can solve the problems of short retention time and large system toxicity, and achieve the effect of prolonging the survival period and high survival rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The preparation of embodiment 1 Sevelamer injection
[0031] Concentration is the preparation of the Sevelamer injection of 5 mg per milliliter, and concrete steps are as follows:
[0032] 1) Take 1500 mL of water for injection, add 27 grams of sodium chloride or 150 grams of glucose to dissolve completely, add 200 grams of hydrochloric acid or sevelamer carbonate raw material, and inert balls (dd15mm:dd5mm:dd2mm:dd0.05mm=50g:50g : 50g: 50g) mixed and put into the reactor.
[0033] 2) Perform ball milling for 2 hours at a speed of 300 r / min with a QM-3SP4 planetary ball mill (Nanjing Nanda Instrument Co., Ltd.).
[0034] 3) After high-temperature sterilization, prepare Sevelamer microspheres and add water to make up to 3000 mL.
[0035] 4) Filling under aseptic conditions, 2mL each, medium boron ampoules, melt-sealed, 1500 in total.
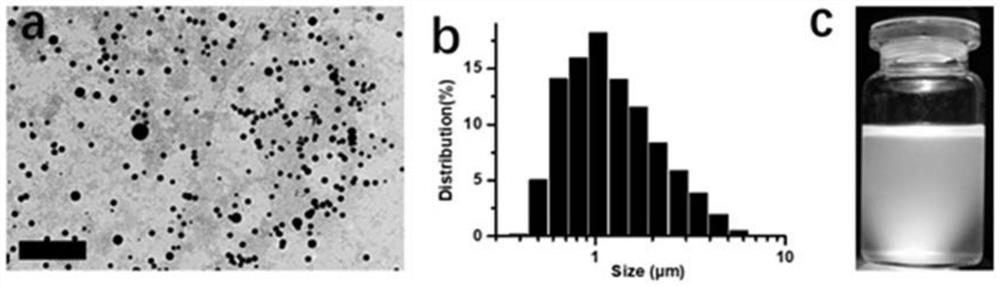
[0036] According to the scanning electron microscope, the size of Sevelamer microspheres after ball milling is between 0.3-7 microns, ...
Embodiment 2
[0037] Example 2 Sevelamer injection to the TASE operation process of the rabbit VX2 liver cancer model
[0038]In this example, the evaluation of TASE treatment was carried out on the rabbit VX2 liver cancer model. The rabbit VX2 liver cancer model is one of the few malignant tumor models with rich blood supply in the liver established in larger animals. The characteristics of the blood supply and imaging findings are very similar. Rabbit VX2 tumor is an ideal experimental model for TACE research of liver cancer, which has a guiding role in clinical practice.
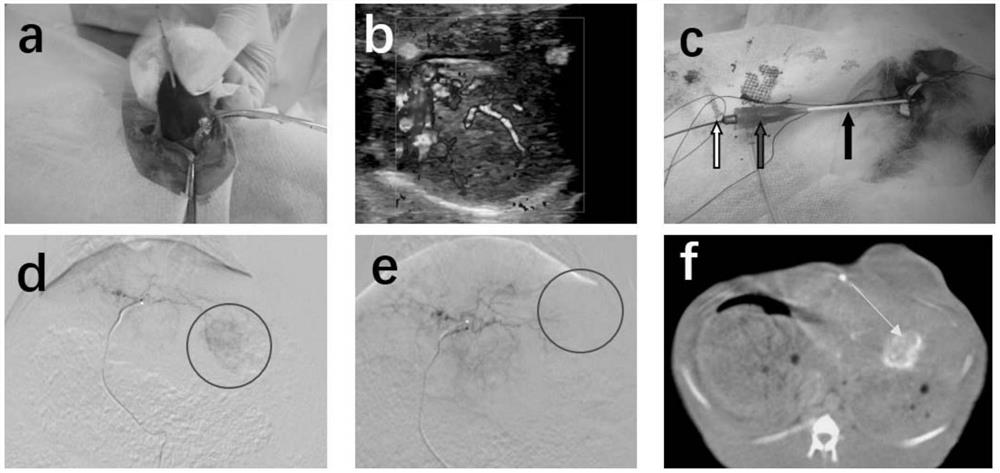
[0039] The TASE operation process of Sevelamer injection on the rabbit VX2 liver cancer model is as follows:
[0040] A. Passage and preservation of VX2 tumor: Homogenate and resuscitate VX2 tumor cells from frozen rabbits preserved in previous studies were inoculated into the lateral muscle group of the right hind limb of healthy New Zealand white rabbits to make tumor-bearing breeding rabbits. Two weeks later, the ...
Embodiment 3
[0045] Embodiment 3 Postoperative treatment evaluation on living body level (imaging)
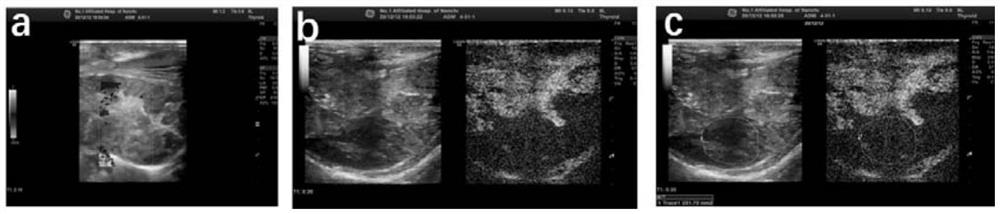
[0046] Ultrasound evaluation 14 days after the operation: Inject 3% amobarbital sodium (1ml / Kg) into the ear vein, fix the rabbit in the supine position on the animal operating table after coma, prepare the skin for disinfection, and lay a sterile towel, routinely Ultrasonography was used to observe tumor size, shape, and internal echo, and whether there was cystic necrosis. Color Doppler flow imaging (CDFI) was used to evaluate the blood supply of the tumor. Contrast-enhanced ultrasound (CEUS) ) to obtain contrast enhancement curves (within 120 seconds after contrast medium injection) of tumor and normal liver tissue at continuous time points to determine the necrosis rate of tumor imaging.
[0047] The result is as image 3 As shown, it can be seen that the tumor area is located near the diaphragm, the boundary is not clear, and the internal echo is uneven. CDFI shows that there is no ob...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com