Electrospinning PLGA/PCL fiber membrane composite chitosan sponge stent for oral cavity alveolar bone regeneration and preparation method thereof
A fiber membrane and chitosan technology, applied in the field of electrospun PLGA/PCL fiber membrane composite chitosan sponge scaffold and its preparation, can solve the problem of active adaptation to the shape of irregular bone defects without reversibility and the degradation speed of filling materials Need to be improved, not suitable for cell growth and other issues, to achieve the effect of preventing penetration, high biocompatibility, and less usage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
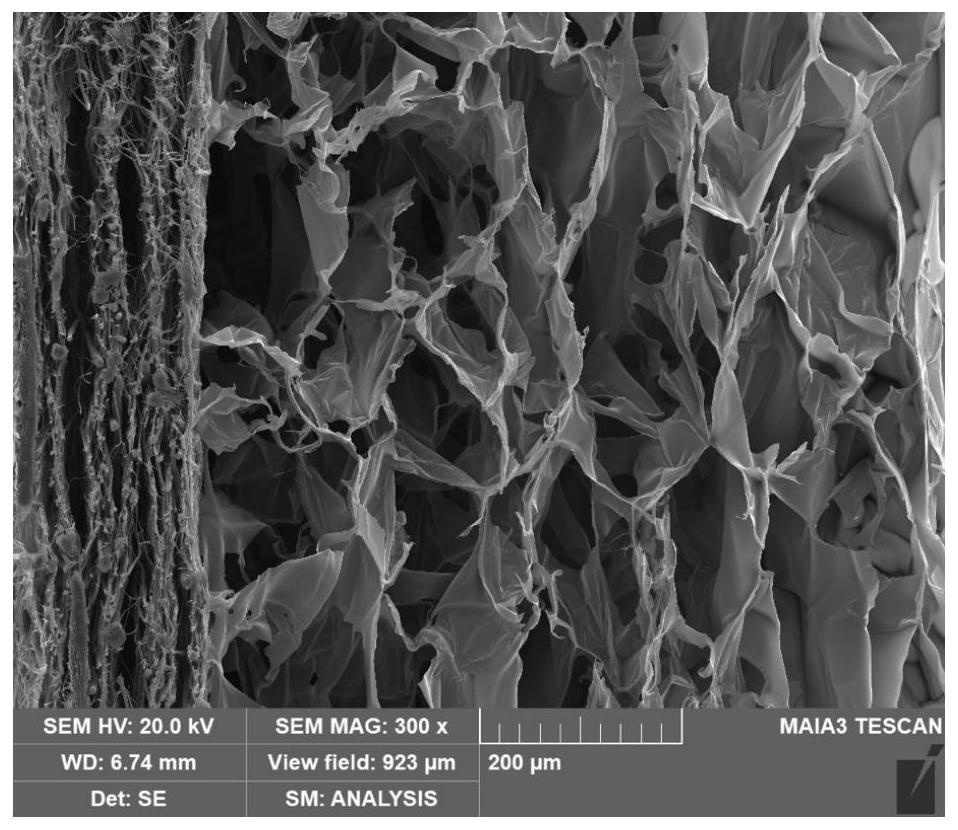
[0047] S1: Dissolving PLGA and PCL in trifluoroethanol at a volume ratio of 1:1 to obtain the first mixed solution, the concentration of PLGA and PCL in the first mixed solution is 0.1g / mL; put the first mixed solution into the syringe, Placed in a microinjector, the injection speed is 0.5mL / h, the voltage of the electrospinning transformer is set to 20kv, the receiving distance is 15cm, the room temperature during electrospinning is 25°C, and the relative air humidity is 35%. The collected fiber membrane Freeze-dried and stored.
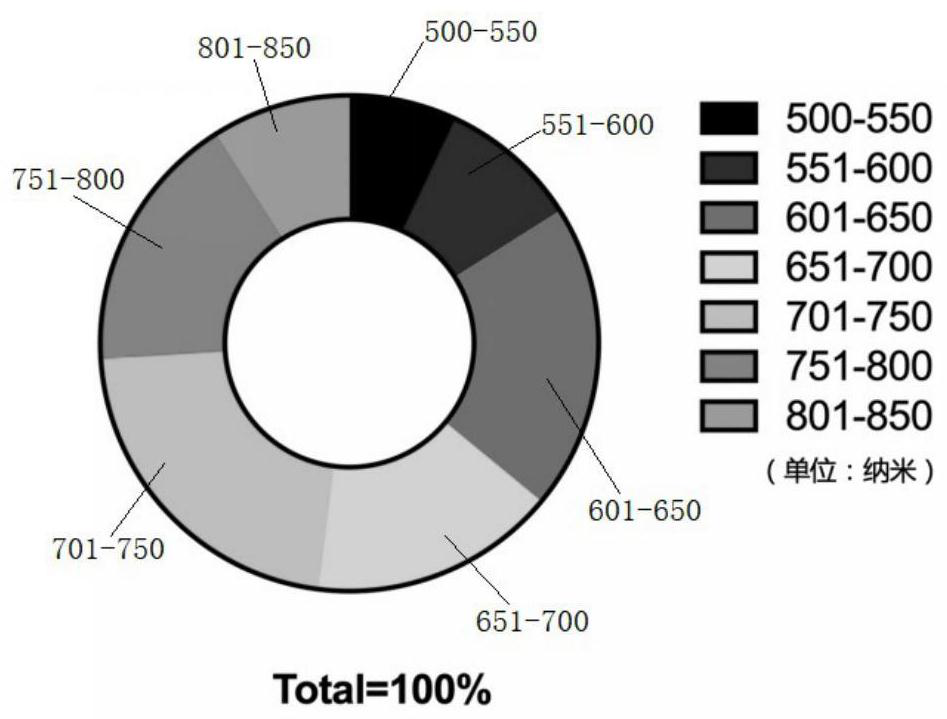
[0048] S2: Dissolve dopamine powder in Tri-HCl solution, stir for 30s to obtain a second mixed solution with a dopamine concentration of 10 mg / mL, the temperature of the second mixed solution is 20°C, and the area is 100-1000mm 2 , the fiber membrane obtained in step S1 with a thickness of 50-500 μm is soaked in the solution in the dark for 2 hours, and the room temperature during soaking is 25°C. After soaking, the fiber membrane is taken out and r...
Embodiment 2
[0053] S1: Dissolving PLGA and PCL in trifluoroethanol at a volume ratio of 2:1 to obtain the first mixed solution, the concentration of PLGA and PCL in the first mixed solution is 0.3g / mL; put the first mixed solution into a syringe, Placed in a microinjector, the injection speed is 0.7mL / h, the voltage of the electrospinning transformer is set to 25kv, the receiving distance is 20cm, the room temperature during electrospinning is 25°C, and the relative air humidity is 50%. The collected fiber membrane Freeze-dried and stored.
[0054] S2: Dissolve dopamine powder in Tri-HCl solution, stir for 60s to obtain a second mixed solution with a dopamine concentration of 15 mg / mL, the temperature of the second mixed solution is 30°C, and the area is 100-1000mm 2 , the fiber membrane obtained in step S1 with a thickness of 50-500 μm was soaked in the solution in the dark for 3 hours, and the room temperature during soaking was 25°C. After soaking, the fiber membrane was taken out and ...
Embodiment 3
[0058] S1: Dissolve PLGA and PCL in trifluoroethanol at a volume ratio of 3:1 to obtain the first mixed solution, the concentration of PLGA and PCL in the first mixed solution is 0.4 / mL; put the first mixed solution into a syringe, place In the microinjector, the injection speed is 0.8mL / h, the voltage of the electrospinning transformer is set to 20kv, the receiving distance is 15cm, the room temperature during electrospinning is 25°C, and the relative air humidity is 20%. The collected fiber membranes are frozen Store dry.
[0059] S2: Dissolve dopamine powder in Tri-HCl solution and stir for 60s to obtain a second mixed solution with a dopamine concentration of 10mg / mL. The temperature of the second mixed solution is 30°C and the area is 100-1000mm 2 , the fiber membrane obtained in step S1 with a thickness of 50-500 μm is soaked in the solution in the dark for 2 hours, and the room temperature during soaking is 25°C. After soaking, the fiber membrane is taken out and rinsed...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com