Anti-hepatitis B virus compound as well as preparation method and application thereof
A compound and anti-hepatitis B technology, applied in antiviral agents, preparation of sugar derivatives, chemical instruments and methods, etc., can solve problems such as drug resistance and unclear drug targets, and achieve low price, high efficacy, and easy synthetic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
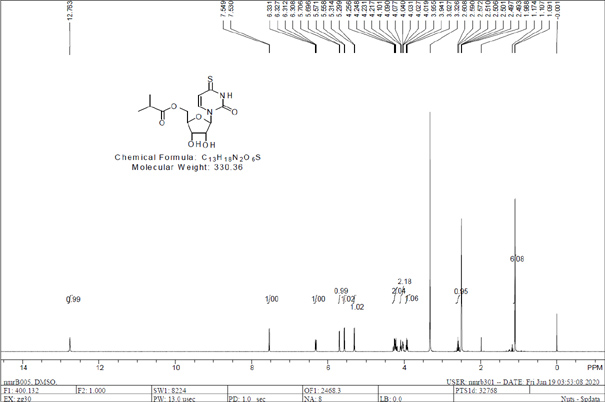
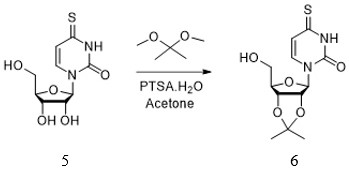
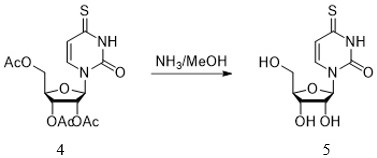
[0043] The invention additionally provides a preparation method of the anti-hepatitis B virus compound. The preparation method includes six steps, and each step can be completed through different embodiments to finally synthesize the compound 4-thiouridine isobutyrate having anti-hepatitis B virus activity. The preparation method of the anti-hepatitis B virus compound comprises the following steps:
[0044] Step 1: the preparation method of compound 3
[0045] .
Embodiment 1
[0047] 25 g of uracil 1, 26.4 g of (2R,3S,4S,5S)-5-(acetoxymethyl)tetrahydrofuran-2,3,4-triacetate triester 2, 48 g of N,O- Bis(trimethylsilyl)acetamide was dissolved in 380 mL of acetonitrile, and reacted under the protection of argon, the oil bath was heated to 90°C and heated to reflux for 2 h, 26 g of trimethylsilyl trifluoromethanesulfonate was added, and continued Heated to reflux for 16h. After the reaction solution was naturally cooled to room temperature, 200 mL of saturated sodium bicarbonate solution and 500 mL of ethyl acetate were added, filtered, the filtrate was separated into the water phase, and the organic phase was washed twice with water and once with saturated saline, and then washed with anhydrous sodium sulfate After drying and filtering, the organic phase was concentrated under reduced pressure to obtain compound 3.
Embodiment 2
[0049] 20g of uracil, 14.09g of (2R,3S,4S,5S)-5-(acetoxymethyl)tetrahydrofuran-2,3,4-triacetate triester, 38.3g of N,O-bis( Dissolve trimethylsilyl)acetamide in 350 mL of acetonitrile, react under the protection of argon, heat the oil bath to 90°C and heat to reflux for 2h, add 20.7g of trimethylsilyl trifluoromethanesulfonate, continue to heat and reflux 16h. The reaction solution was naturally cooled to room temperature, added 150 mL saturated sodium bicarbonate solution and 450 mL ethyl acetate, filtered, the filtrate was separated into the water phase, the organic phase was washed twice with water, once with saturated brine, and dried over anhydrous sodium sulfate , after filtration, the organic phase was concentrated under reduced pressure to obtain compound 3.
[0050] Further, the compound 3 obtained in Example 1 and Example 2 are both yellow oils, and the crude compound 3 obtained in Example 1 and Example 2 does not need to be purified, and can directly participate in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com