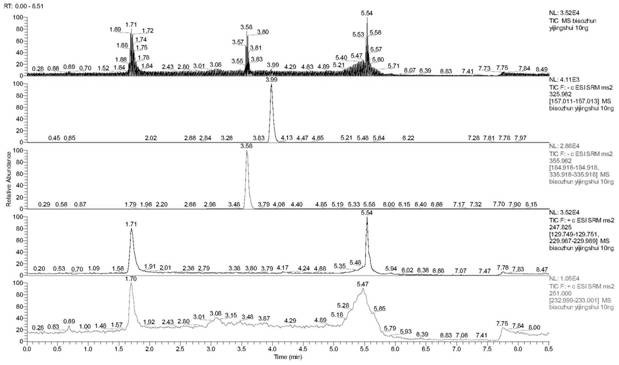
Method for rapidly detecting florfenicol and florfenicol amine in honey
The technology of florfenicol and florfenicol is applied in the field of rapid detection of florfenicol and florfenicol in honey, and can solve the problems of long test period, complicated pretreatment of solid-phase extraction column, and the like, To achieve the effect of simple and fast detection operation, good linear relationship, accurate qualitative and quantitative analysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] A kind of rapid detection method of florfenicol and florfenicol amine in honey, comprises the steps:
[0031] S1, standard solution preparation;
[0032] S1.1. Standard stock solution: Accurately weigh 10.0 mg of florfenicol, florfenicol amine, and chloramphenicol-d5 standard substance respectively, dissolve in methanol and dilute to 10 mL, and the mass concentration of the standard stock solution is 1.0 mg / mL, store in the dark below -20°C, and reserve, the purity of florfenicol, florfenicol amine and chloramphenicol-d5 is >99.0%;
[0033] S1.2. Standard intermediate solution: Accurately measure 100 μL of florfenicol and florfenicol amine standard stock solution respectively, dilute to 10 mL with methanol, the mass concentration of the standard intermediate solution is 10 μg / mL, and the internal standard standard intermediate solution: Accurately measure 100 μL of chloramphenicol-d5 standard stock solution, dilute to 10 mL with methanol, and the mass concentration of ...
Embodiment 2
[0045] 1. Materials and methods
[0046] 1.1 Reagents
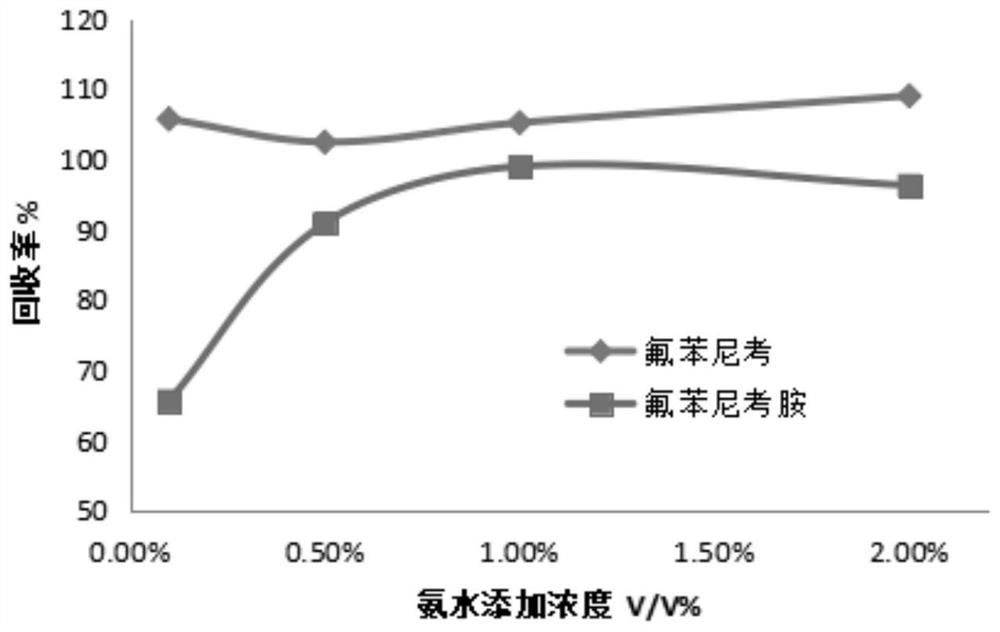
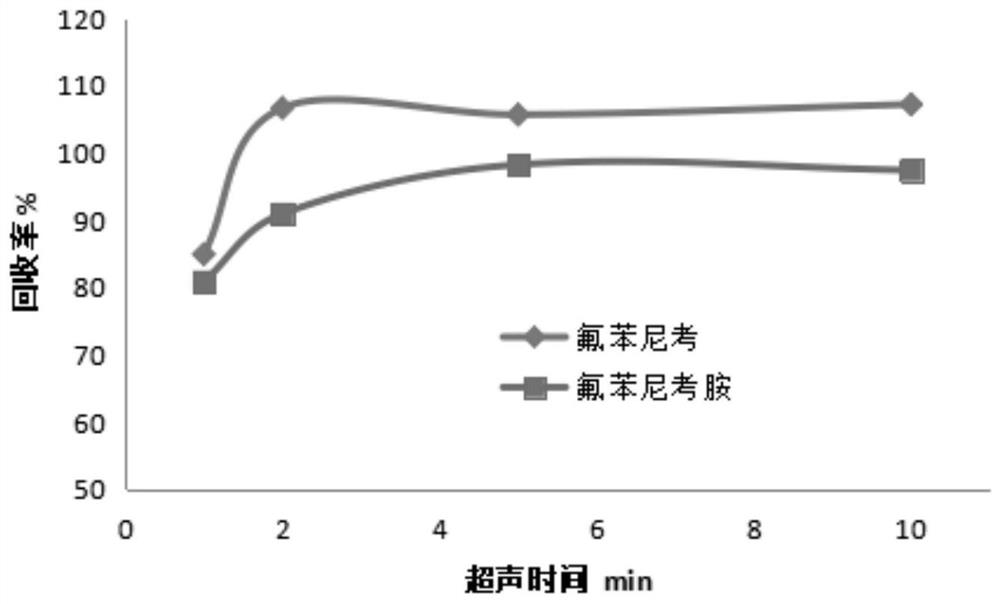
[0047] Florfenicol and florfenicol amine standard products, and D5-chloramphenicol standard product were purchased from Dr. Ehrenstorfer GmbH in Germany, with a purity >99.0%; D3-florfenicol amine standard solution, with a fixed concentration of 5 mg / L, The customization unit is the Agricultural Product Quality Standard Research Center of the Ministry of Agriculture and Rural Affairs. Methanol, formic acid, acetonitrile, ethyl acetate, and acetone are chromatographically pure reagents from Merck, Germany; ammonia water is analytically pure reagents from Sinopharm. PSA and C18 solid phase extraction materials were purchased from Agilent Technologies Co., Ltd.;
[0048] For the selection of purification materials, the influence of purification materials on the recovery efficiency was investigated experimentally, and the purification effect was determined by the recovery rate of the negative honey sample added with the sta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com