Preparation and application of 1, 8-naphthalimide hydrogen sulfide fluorescent molecular probe
A technology of fluorescent molecular probes and naphthalimides, which is applied in the fields of fluorescence/phosphorescence, chemical instruments and methods, and material analysis through optical means. It can solve the problems of limited application and narrow linear range, and achieve economical and cheap High sensitivity, good specific effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
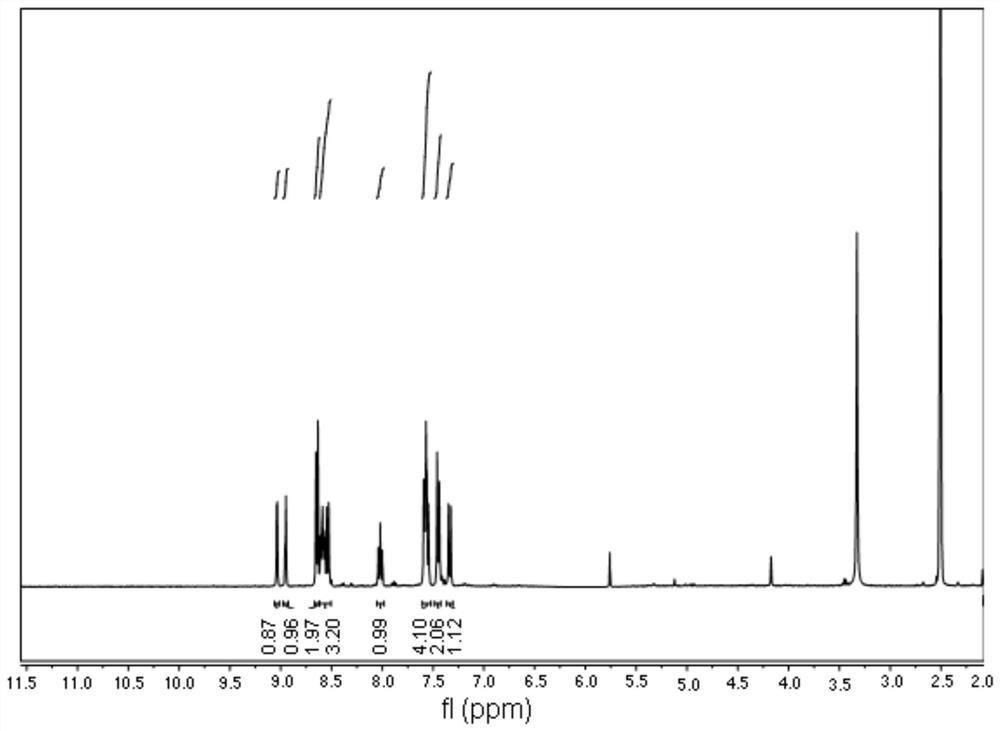
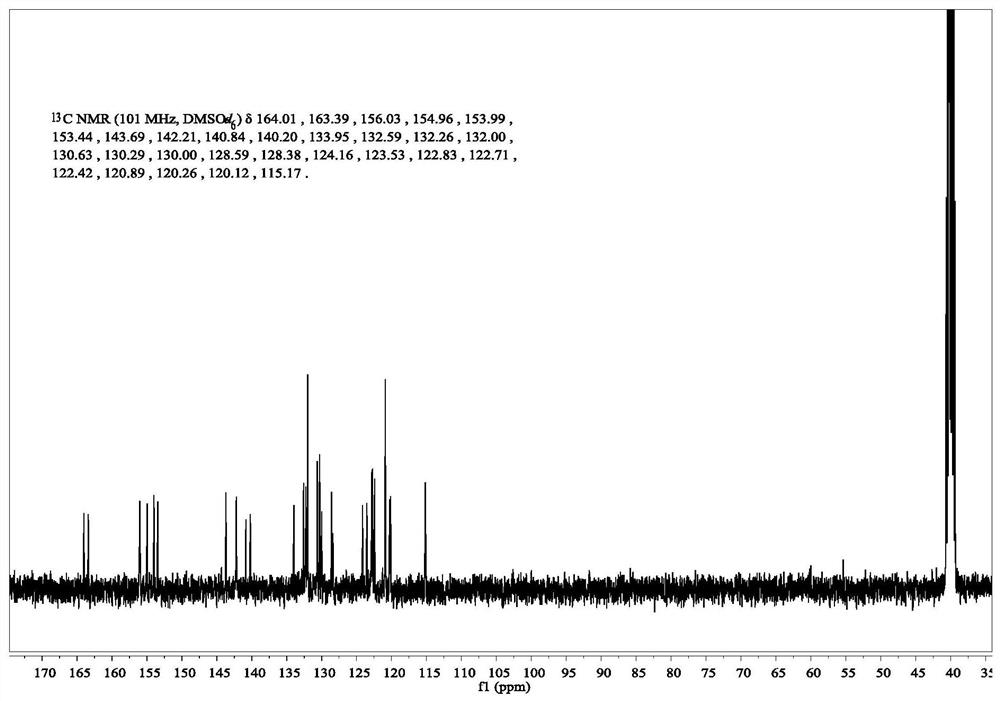
[0041] Example 1: 1,8-naphthalimide H 2 Synthesis of S Fluorescent Molecular Probes
[0042] 1. Synthesis of N-(4-hydroxyphenyl)-4-bromo-1,8-naphthalimide (intermediate compound 1a):
[0043] Under the protection of nitrogen, 2.77g (10.00mmol) of 4-bromo-1,8-naphthalene dicarboxylic anhydride was dissolved in 50mL of absolute ethanol, and after stirring at room temperature, 1.31g (12.00mmol) of 4-aminophenol was added, Stirring was continued and the temperature of the reaction liquid was raised to 80° C., and the reaction was refluxed for 8 hours. After the complete conversion of the raw material was monitored by TLC (the developer was petroleum ether: ethyl acetate = 2:1, V / V), the heating was stopped, and the reaction liquid was cooled down. During the cooling process, solids could be observed to precipitate out gradually. Suction filtration, washing, and thorough drying afforded 3.34 g of a brown solid, with a yield of 90.76%.
[0044] 2. Synthesis of N-(4-hydroxyphenyl)...
Embodiment 2
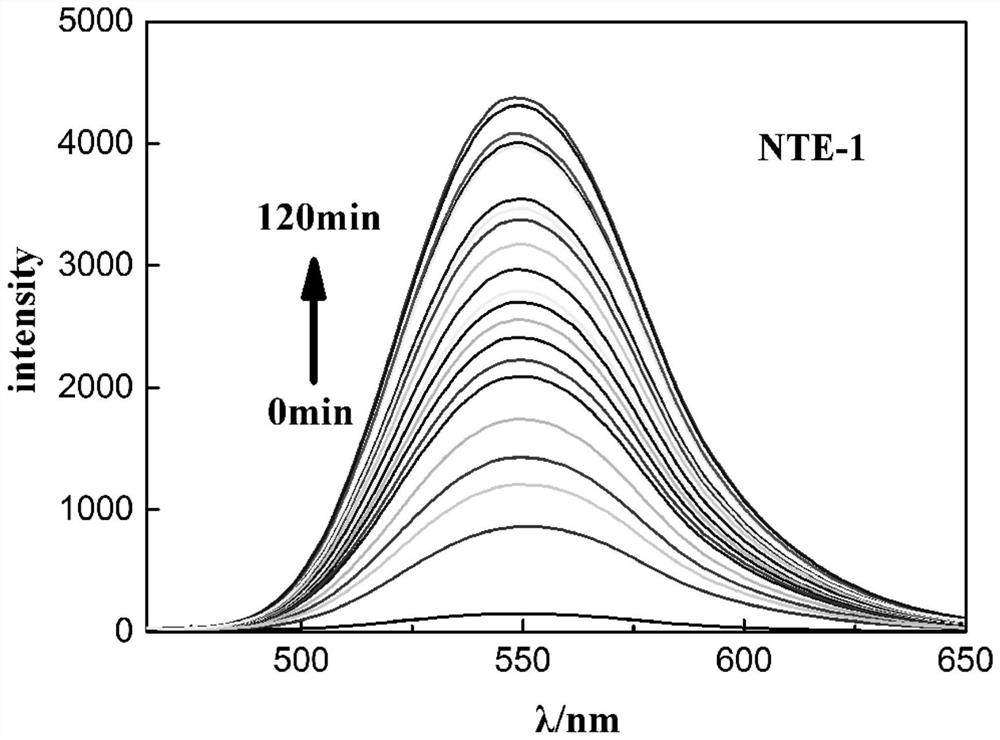
[0055] Example 2: 1,8-naphthalimide H 2 Application of S Fluorescent Molecular Probes
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com