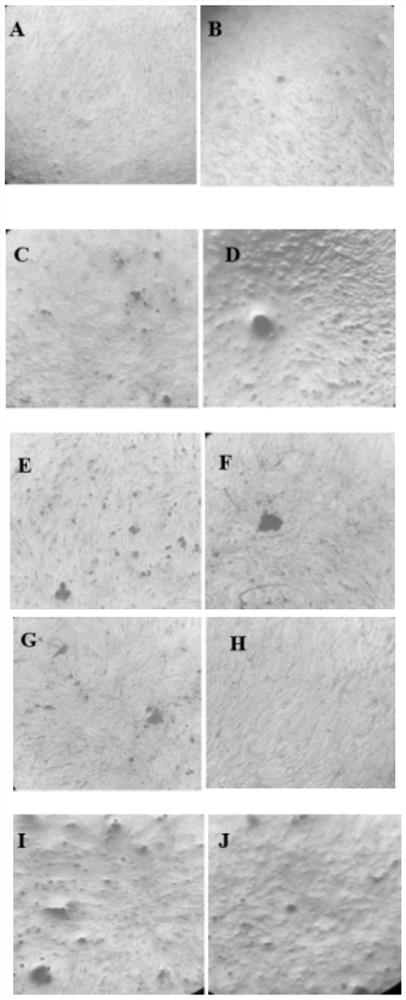
Phosphate ester derivative of herba epimedii as well as preparation method and application of phosphate ester derivative
A technology of phosphates and epimedium, applied in the field of phosphate derivatives of epimedium and its preparation, can solve the problems of obvious side effects and restriction of drug use, and achieve simple preparation process, low cost, and enhanced cytotoxicity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
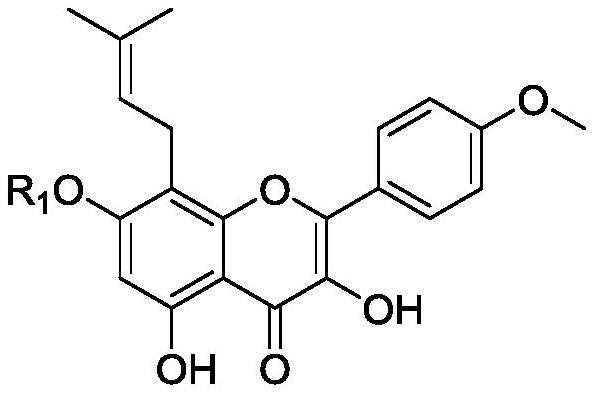
[0034] 3,5,7-Trihydroxy-8-(3-methyl-2-butenyl)-2-(4-methoxyphenyl)-7-(4H-benzopyran-4-one) (Icariin), the structure of icariin is shown below:
[0035]
[0036] Since icariin is less present in Epimedium, icariin is extracted and isolated from the traditional Chinese medicine Epimedium, mainly obtained by hydrolyzing icariin. Icariin uses icariin as raw material, and is hydrolyzed by immobilized α-L-rhamnosidase and glucosidase. Take 5 g of icariin and add 50 mL of deionized water to a 250-mL round-bottomed flask, place it in a constant temperature water bath shaker at 60 °C, adjust the pH to 9-10, and add immobilized α-L-rhamnosus after the substrate is dissolved. 1 g of glycosidase and 1 g of glucosidase were reacted for 3 hours at an oscillation frequency of 120 r / min, monitored by TLC, after the reaction of the raw materials was completed, the pH was adjusted to 3-4, the filtrate was placed in a refrigerator until it was precipitated, and the epimedium was obtained by ...
Embodiment 2
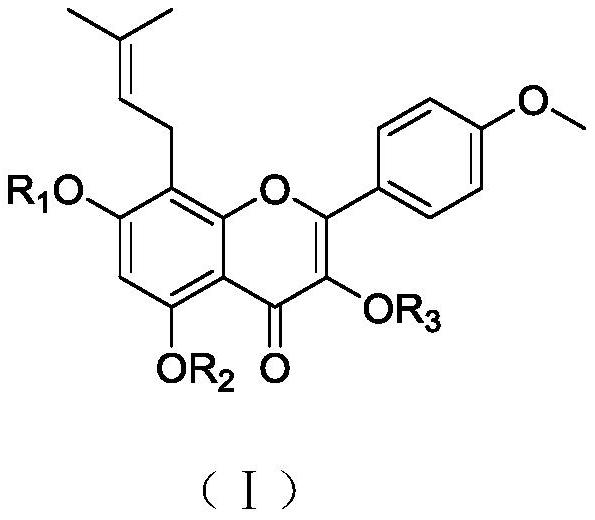
[0040] 3,5-Dihydroxy-8-(3-methyl-2-butenyl)-2-(4-methoxyphenyl)-7-(4H-benzopyran-4-one)phosphoric acid bis The structural formula of methyl ester is:
[0041]
[0042]3,5,7-trihydroxy-8-(3-methyl-2-butenyl)-2-(4-methoxyphenyl)-7-(4H-benzopyridine) synthesized in Example 1 Furan-4-one) (icariin) (193 mg, 0.5 mmol) was dissolved in 20 mL of tetrahydrofuran, and the catalysts were added diisopropylethylamine (DIPEA, 0.348 mL, 2 mmol) and 4-dimethylaminopyridine (DMAP, 6 mg) , 0.05mmol), cooled to 0°C in an ice-water bath, slowly added CCl of dimethyl phosphite (46uL, 0.5mmol) 4 (2 mL), slowly raised to 20°C, and the reaction was monitored by thin layer chromatography (methanol:dichloromethane=1:10). After the reaction was completed, it was concentrated under reduced pressure, and purified by column chromatography (silica gel column purification) to obtain the target compound 3,5-dihydroxy-8-(3-methyl-2-butenyl)-2-(4-methyl) Oxyphenyl)-7-(4H-benzopyran-4-one) dimethyl phosph...
Embodiment 3
[0046] 3,5-Dihydroxy-8-(3-methyl-2-butenyl)-2-(4-methoxyphenyl)-7-(4H-benzopyran-4-one)phosphoric acid bis The structural formula of ethyl ester is:
[0047]
[0048] 3,5,7-trihydroxy-8-(3-methyl-2-butenyl)-2-(4-methoxyphenyl)-7-(4H-benzopyridine) synthesized in Example 1 Furan-4-one) (icariin) (193 mg, 0.5 mmol) was dissolved in tetrahydrofuran (20 mL), and the catalysts were added diisopropylethylamine (DIPEA, 0.348 mL, 2 mmol) and 4-dimethylaminopyridine (DMAP). , 6 mg, 0.05 mmol), cooled to 0 °C in an ice-water bath, and slowly added diethyl phosphite (64 uL, 0.5 mmol) in CCl 4 (2 mL), slowly warmed to 20°C, and the reaction was monitored by thin layer chromatography (methanol:dichloromethane=1:10). After the reaction was completed, the reaction was concentrated under reduced pressure, and purified by column chromatography (silica gel column purification) to obtain the target compound 3,5-dihydroxy-8-(3-methyl-2-butenyl)-2-(4-methoxy phenyl)-7-(4H-benzopyran-4-one) d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com