Method for identifying avian leukosis virus and chicken infectious anemia virus through visual double LAMP
A technology for avian leukosis virus and chicken infectious anemia, which is applied in biochemical equipment and methods, recombinant DNA technology, and microbial determination/inspection, etc., and can solve the problems of increased amplicon contamination risk, deviation of Tm value, laboratory contamination, etc. problem, to achieve the effect of good specificity, low pollution and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Embodiment 1, the design of double LAMP primer and the preparation of positive plasmid
[0096] 1. Design of primers and probes
[0097] According to the conserved sequence of the pol gene and CIAV VP2 gene of ALV (subtypes A, B, and J) in GenBank, LAMP primers were designed using MEGA 4.0 and Primer Explorer V4 online primer design software, and the outer primers included ALV-F3, ALV-B3 , CAV-F3 and CAV-B3, internal primers include ALV-FIP, ALV-BIP, CIAV-FIP and CIAV-BIP, internal primers FIP=F1c+F2, BIP=B1c+B2; Utilize PrimerPrimer5.0 in ALV and Two probes were designed between F1c and B1c of CIAV, respectively ALV-probe, 5' end labeled with FAM fluorophore, 3' end labeled with BHQ3 quencher group; CIAV-Probe, 5' end labeled with CY 5 fluorophore group, the 3' end is labeled with the BHQ3 quencher group. The sequences of primers and probes are shown in Table 1. The sequences of inner primers and outer primers were synthesized by Huada Gene Biotechnology (Shenzhen) C...
Embodiment 2
[0110] Embodiment 2, establishment of double LAMP method
[0111] 1. Reaction system
[0112] 2 μL DNA / cDNA template, 10 μL 2×Reaction Mix, 0.8 μL Bst DNApolymerase, 0.8 μL each of internal primers ALV-FIP, ALV-BIP, CIAV-FIP and CIAV-BIP (both working concentrations are 40 μmol / L), external primer ALV -0.4 μL each of F3, ALV-B3, CIAV-F3 and CIAV-B3 (working concentration is 5 μmol / L), 0.4 μL of ALV-Probe (working concentration is 0.5 μmol / L), CIAV-Probe (working concentration is 0.5 μmol / L) 0.8μL plus ddH 2 After making up 20 μL of O, the reaction tube was placed in a thermostat or a Loopamp LA-320C real-time turbidimeter to react at 62°C for 60 minutes, and inactivated at 80°C for 5 minutes. After the test, the reaction tube was directly placed in a multicolor fluorescence imaging system for determination.
[0113] 2. Visual double LAMP result judgment
[0114] Visual double LAMP After judging the amplification result by monitoring the turbidity with a real-time turbidimet...
Embodiment 3
[0115] Embodiment 3, specificity test
[0116] Samples to be tested: positive ALV (mixed template of A subgroup, B subgroup and J subgroup), CIAV, ALV and CIAV mixed template, FAdV-4, ARV, NDV, AIV-H5, AIV-H7, AIV-H9 , AEV, REV, IBV, IBDV and MDV cDNA or DNA templates.
[0117] Using the sample to be tested as a template, perform double LAMP amplification according to the method established in Example 2.
[0118] Set RNase-free water as a negative control.
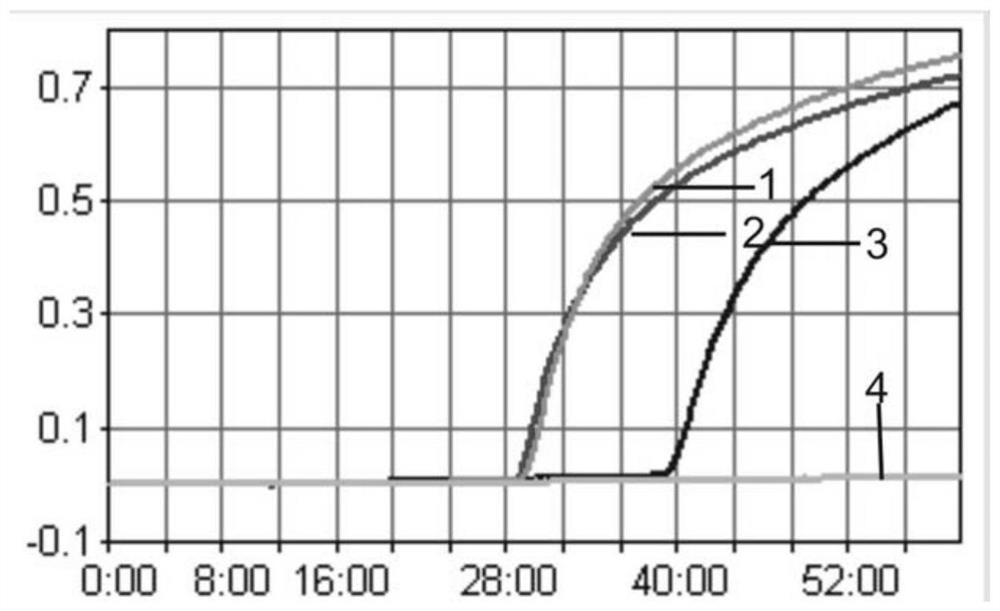
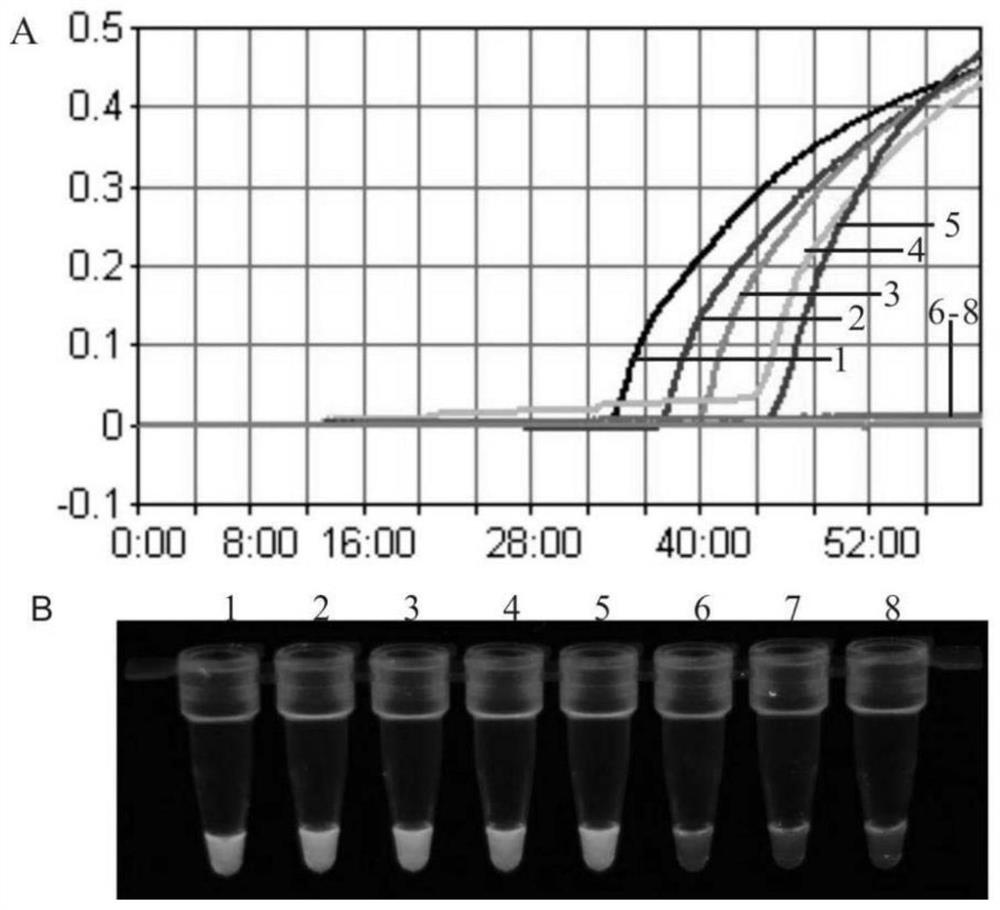
[0119] The cDNA of positive ALV and the DNA of CIAV are used as templates, RNase-free water is set as a negative control, and the reaction tube is placed in a Loopamp LA-320C real-time turbidimeter to react, and the results show that the cDNA of ALV and the DNA of CIAV are amplified ( figure 1 ). The positive ALV (mixed template of A subgroup, B subgroup and J subgroup), CIAV, ALV and CIAV mixed template, FAdV-4, ARV, NDV, AIV-H5, AIV-H7, AIV-H9, AEV, cDNA or DNA of REV, IBV, IBDV and MDV were used as templates, and RN...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com