Efficient retarded acidification working fluid and preparation method thereof
A working fluid and retarding technology, which is applied in chemical instruments and methods, earthwork drilling, wellbore/well components, etc., can solve the problems of weak corrosion, achieve easy flowback, improve core permeability performance, prolong acid The effect of rock reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Preparation of the first high-efficiency slow-acidizing working solution.
[0021] Set the mass of the high-efficiency slow acidifying working solution to be configured as 100 parts; under the conditions of normal temperature and pressure, first use tap water and ammonium bifluoride to prepare an ammonium salt solution with a mass fraction of 10%; then add a quantitative amount to the ammonium salt solution in turn hydrochloric acid, hydroxyethylene diphosphonic acid and ammonium bifluoride, stir and mix evenly to obtain a retarder with a mass fraction of hydrochloric acid of 12%, a mass fraction of hydroxyethylene diphosphonic acid of 15%, and a mass fraction of ammonium bifluoride of 10% acid solution; then add quantitatively self-developed Mannich base corrosion inhibitor, clay stabilizer ammonium chloride and iron ion stabilizer ethylenediaminetetraacetic acid into the retarding acid solution, stir and mix evenly to obtain a hydrochloric acid solution. The mass frac...
Embodiment 2
[0023] Preparation of the second high-efficiency slow-acidizing working solution.
[0024] The mass fraction of the acidifying working solution to be prepared is set to 100; under the conditions of normal temperature and pressure, tap water and ammonium fluoride are used to prepare an ammonium salt solution with a mass fraction of 15%; then hydrochloric acid and hydroxyl groups are added to the ammonium salt solution in sequence Ethylene diphosphonic acid and dihexene triamine pentamethylidene phosphonic acid (mass fraction of 1:1), stir and mix evenly to obtain hydrochloric acid with a mass fraction of 12%, hydroxyethylene diphosphonic acid and dihexyl A retarded acid solution with a mass fraction of enetriamine pentamethylene phosphonic acid of 15% and a mass fraction of ammonium fluoride of 15%; and then quantitatively self-developed quaternary ammonium salts and imidazole retarders were added to the retarded acid solution. Etching agent, clay stabilizer potassium chloride ...
Embodiment 3
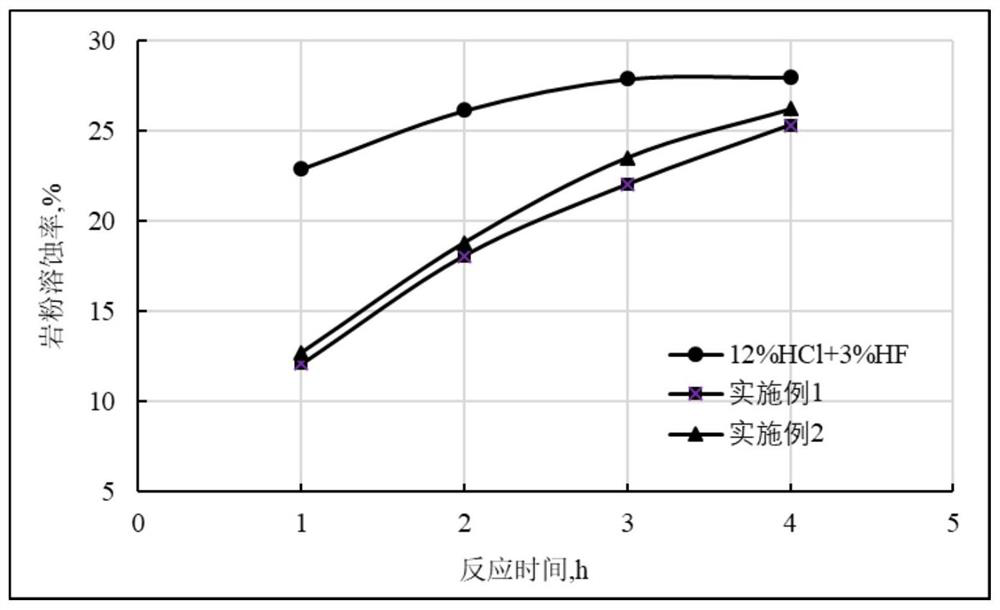
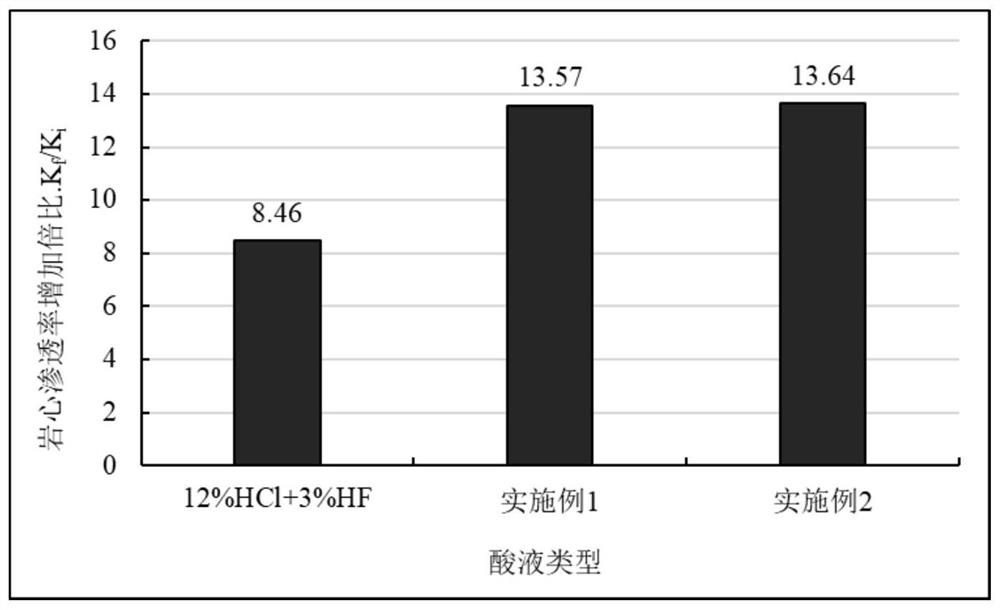
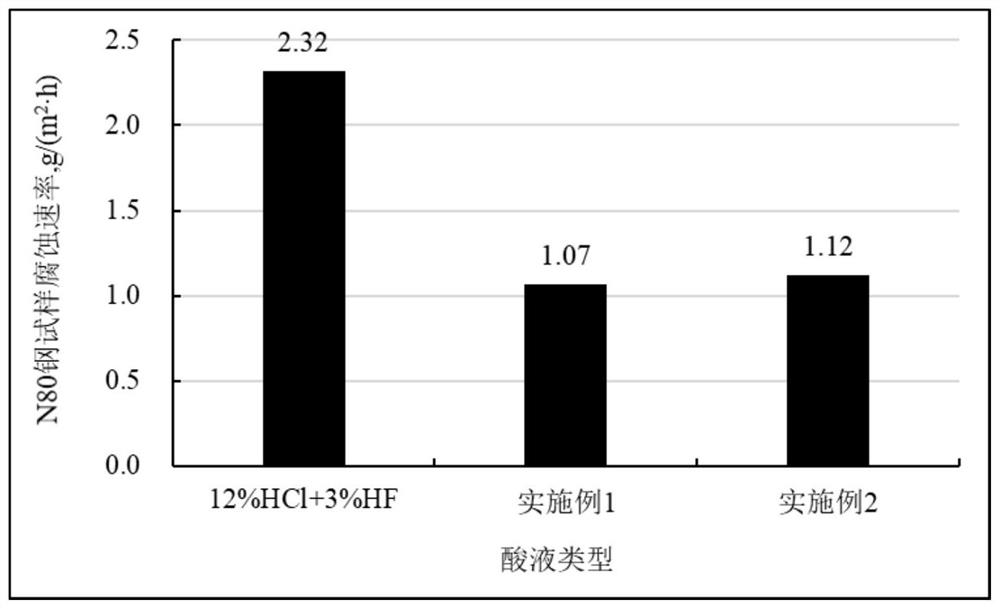
[0026] Experiment on the dissolution rate of high-efficiency slow-acidizing working fluid on rock powder.
[0027] The rock powder was dried at 80 °C for 4 h, and then placed in a drying bottle and sealed for later use. The dried rock powder was taken out and weighed at room temperature, and the mass of each part was 5.0000 g. According to the dosage of 1g of rock powder acid solution, 10ml, put rock powder and different acid solutions into a wide-mouth bottle, shake to make the acid solution and rock powder fully contact, and then let stand. After drying for 6h, the filter paper and the residue were weighed. The dissolution rate of the rock powder was calculated according to the mass of the rock powder before and after the reaction, and was calculated by formula (1). The calculation results are shown in Table 1. All waste liquids in this experiment were poured into waste liquid recycling buckets for unified treatment.
[0028]
[0029] where m 1 is the initial mass of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com