Ciclosporin solid dispersion and tablet preparation method thereof
A solid dispersion and solid dispersion technology, applied in the direction of cyclic peptide components, medical preparations of non-active ingredients, pill delivery, etc., can solve the problems of rare application of polypeptide drugs, achieve good recrystallization inhibition, and in vivo blood medicine The effect of high concentration and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
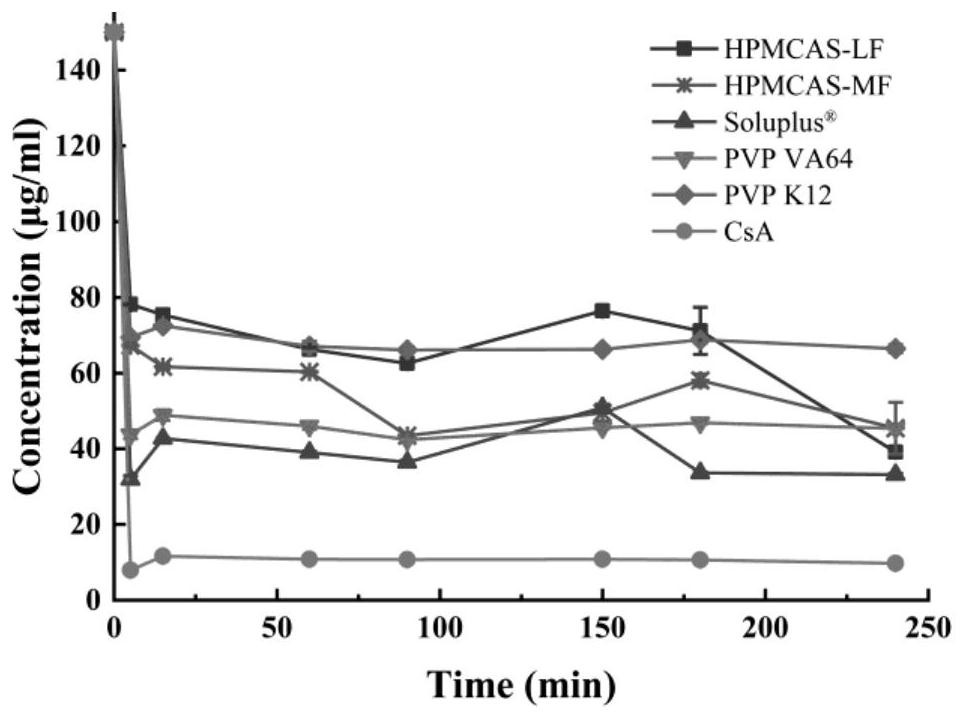
[0029] Experimental Example 1 prepared 50 mL of each polymer solution with a concentration of 100 μg / mL (the polymer includes HPMCAS-LF, HPMCAS-MF, PVP VA64, PVP K12), quantitative excess CsA was added to the above solution respectively, and as a blank control, each sample was measured in duplicate, and then the solubilization of each polymer to CsA was measured under different pH conditions. Determination conditions: temperature 25°C, rotation speed 150rpm / min, shaker 24h, UV method to determine the concentration of CsA.
[0030] The results are shown in Table 1, PVP K12, and PVP VA64 have strong solubilizing ability, while HPMCAS-LF and HPMCAS-MF have almost no solubilizing effect. At the same time, it can be seen that the dissolution behavior of CsA has no pH dependence.
[0031] Table 1
[0032]
experiment example 2
[0033] Experimental Example 2 Weighed an appropriate amount of CsA raw material and dissolved it in the least amount of organic solvent (3.5 mL of methanol) to form a uniform supersaturated solution to be crystallized to simulate the supersaturated drug system of CsA. Weigh 70mg of each polymer, add 700mL of medium with pH 6.8 to prepare a solution with a concentration of 100μg / mL, duplicate in parallel and make a blank control. The supersaturated drug solution was added to the above polymer solution, and the experiment was carried out at 37° C. and 50 rpm / min by using the paddle method. At different sampling time points, 4 mL samples were taken, filtered through a 0.45 μm filter membrane, and the subsequent filtrate was taken. The absorbance was measured by UV method, and the drug concentration was calculated.
[0034] Such as figure 1 As shown, in the blank control without adding polymer, the drug crystallized rapidly within 5 minutes, and was always in a low concentration ...
Embodiment 1
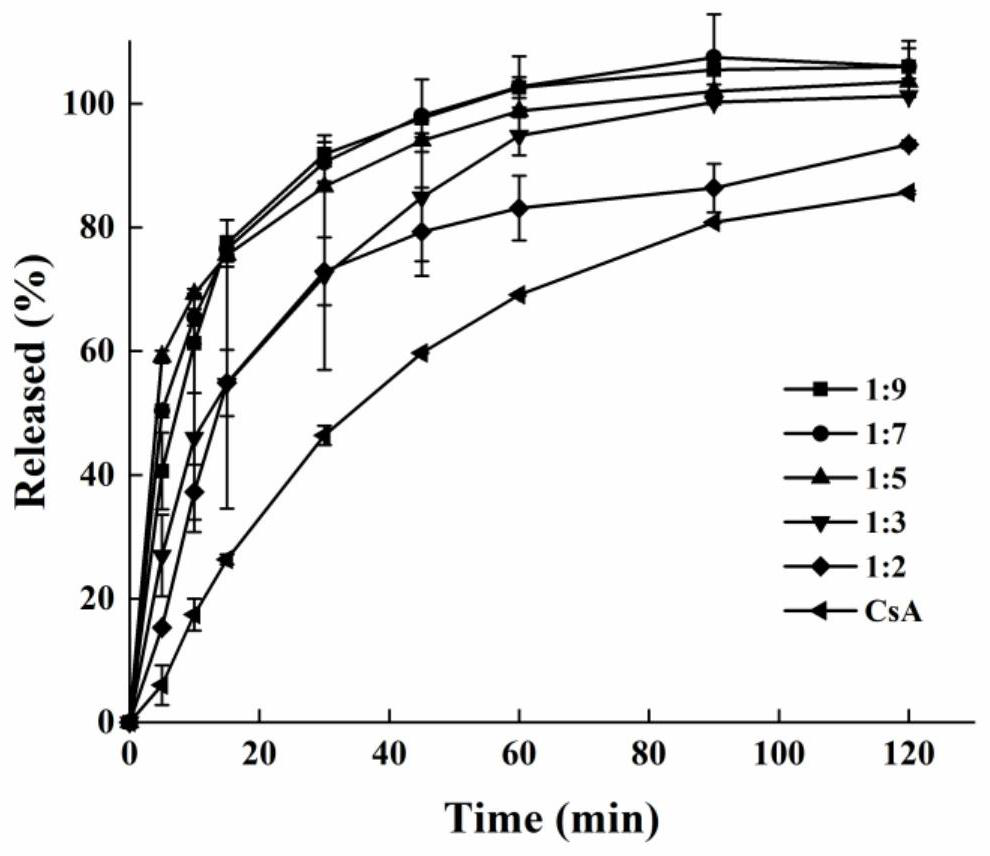
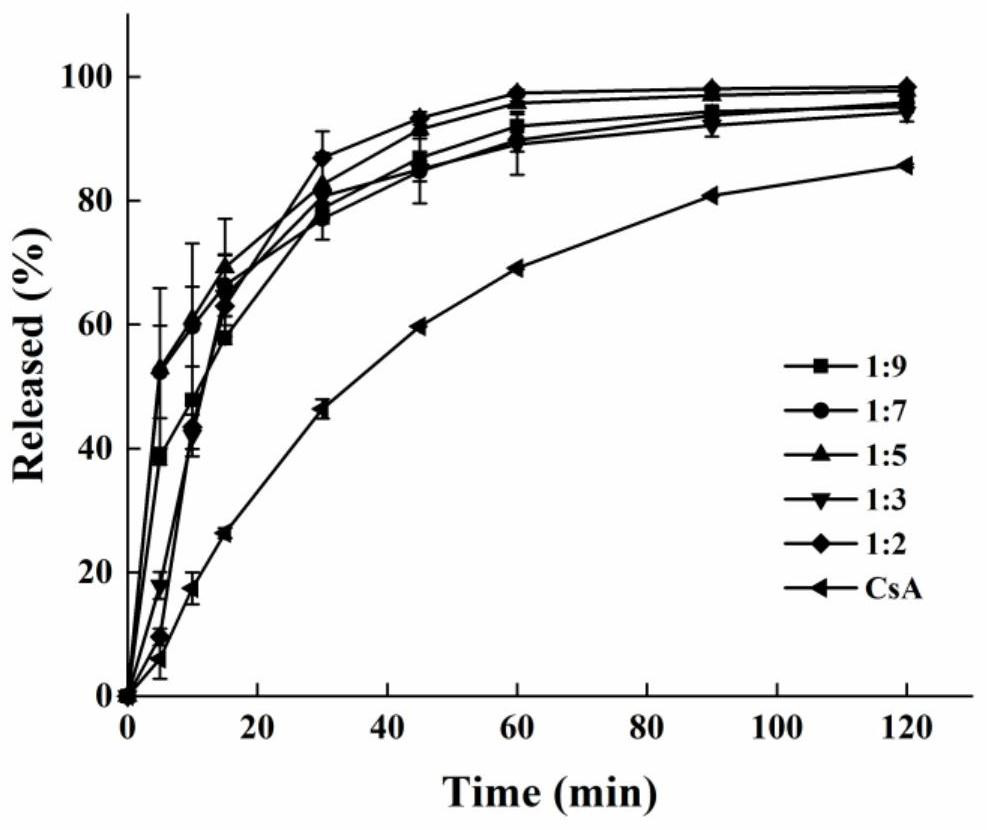
[0035] Example 1 Mix cyclosporine and PVP K12 uniformly at a ratio of 1:2 to prepare a physical mixture. Set the extrusion temperature at 140°C and the rotation speed at 30rpm. After the temperature rises to the set value and stabilizes, add the physical mixture at a constant speed , to obtain strip-shaped extrudates, cooled, and crushed through an 80-mesh sieve to obtain cyclosporine solid dispersion powder for in vitro dissolution experiments.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com