Ceramide compound as well as cationic liposome, preparation method and application thereof
A technology of cationic liposomes and ceramides, applied in the field of biomedicine, can solve problems that have not been reported before, and achieve the effects of good passage, strong targeting, and strong fat solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] The preparation of embodiment 1 ceramide
[0065] 0.15 g of 10-hydroxyoctadecanoic acid (0.5 mmol) was dissolved in 5 mL of dichloromethane, 0.848 g of Dess-Martin oxidant (DMP, 2 mmol) and 0.84 g of sodium bicarbonate (10 mmol) were added. The mixed solution was stirred overnight at room temperature (25±5°C). Add 20 mL of saturated sodium thiosulfate to the mixed solution and continue to stir for 2 hours, then extract with ethyl acetate, dry the organic phase over anhydrous magnesium sulfate, concentrate under reduced pressure, and separate and purify the crude product by flash column chromatography to obtain 10-carbonyl octadecanoic acid ;
[0066] Collect 0.15 g of 10-carbonyl octadecanoic acid (0.5 mmol), dissolve it in 5 mL of methanol, then add 0.616 g of ammonium acetate (8 mmol), and react at room temperature for 1.5 hours. Add 0.24g sodium cyanoborohydride (5mmol) to the mixed solution, stir for 2 days, then extract with ethyl acetate, dry the organic phase o...
Embodiment 2
[0071] The preparation of embodiment 2 ceramide
[0072] 0.15 g of 10-hydroxyoctadecanoic acid (0.5 mmol) was dissolved in 5 mL of chloroform, 0.424 g of Dess-Martin oxidizer (DMP, 1 mmol) and 1.68 g of sodium bicarbonate (20 mmol) were added. The mixed solution was stirred overnight at room temperature. Add 5.772 mL of saturated sodium thiosulfate to the mixed solution and continue to stir for 3 hours, then extract with ethyl acetate, dry the organic phase over anhydrous magnesium sulfate, concentrate under reduced pressure, and separate and purify the crude product by flash column chromatography to obtain 10-carbonyl octadecadeca Carbonic acid;
[0073] Collect 0.15 g of 10-carbonyl octadecanoic acid (0.5 mmol), dissolve it in 5 mL of isopropanol, then add 0.385 g of ammonium acetate (5 mmol), and react at room temperature for 1.5 hours. Add 0.288g sodium cyanoborohydride (6mmol) to the mixed solution, stir for 2 days, then extract with ethyl acetate, dry the organic phase...
Embodiment 3
[0078] The structure identification of embodiment 3 ceramide
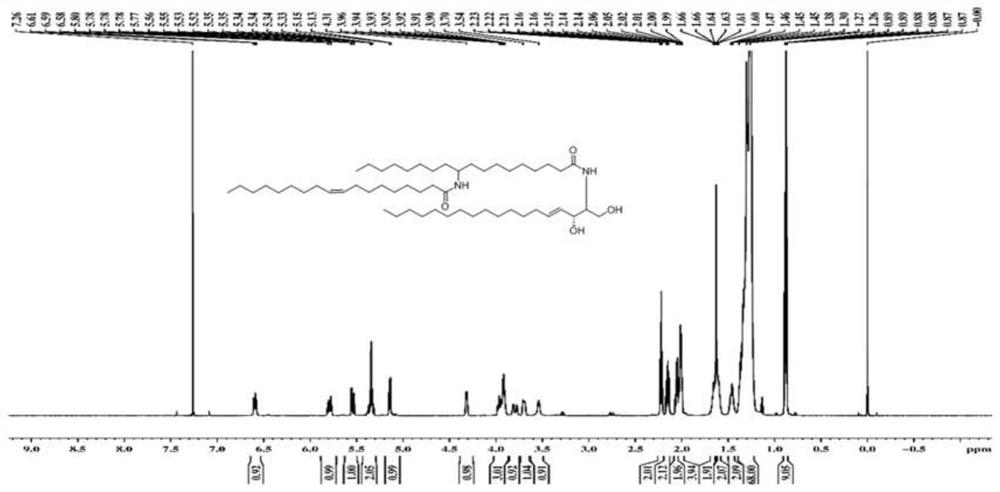
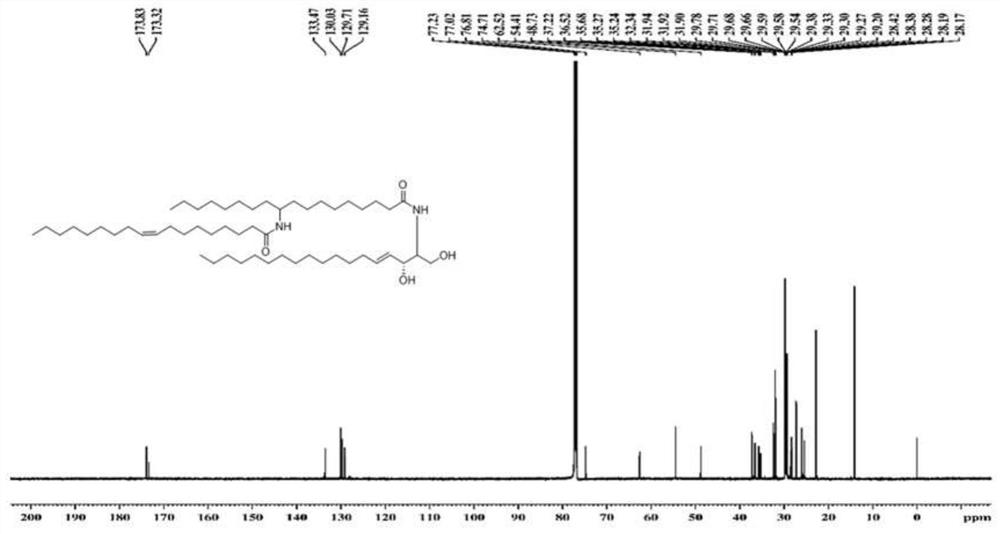
[0079] Utilize nuclear magnetic resonance spectrum to measure the ceramide structure that embodiment 1 prepares, obtain corresponding proton nuclear magnetic resonance spectrum figure and carbon nuclear magnetic resonance spectrum figure, as figure 1 and 2 As shown, the ceramide has a structural formula shown in Formula I from the spectrogram results. The nuclear magnetic resonance spectroscopy determination method is a conventional technical means in the art, and will not be repeated here.
[0080] 1 H NMR (600MHz, CDCl 3 ): δ6.50(d, J=7.1Hz, 1H), 5.78(dt, J=15.1, 6.9Hz, 1H, H-5), 5.53(dd, J=15.1, 5.5Hz, 1H, H-4 ),5.31-5.38(m,2H),5.18(d,J=9.3Hz,1H),4.30-4.31(m,1H),3.90-3.97(m,3H),3.68-3.70(m,2H), 3.48(b,1H),2.23(t,J=7.5Hz,2H),2.15(q,2H),2.05(q,2H),2.01(q,4H),1.61-1.65(m,4H),1.42 -1.47(m,2H),1.26-1.36(m,64H),0.87-0.89(m,9H).
[0081] 13 C NMR (150MHz, CDCl 3 ): δ173.8, 173.0, 133.6, 130.0, 129.7, 129.1, 74...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| surface potential | aaaaa | aaaaa |
| surface potential | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com