Preparation method of Ag/alpha-Co(OH)2 oxygen evolution catalyst
A catalyst and oxygen evolution technology, applied in the direction of metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, chemical instrument and method, etc., can solve the problem of high manufacturing cost, limitation of large-scale production and application, Poor stability and other problems, to achieve the effect of convenient operation, improved catalyst performance and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1)α-Co(OH) 2 Synthesis of Nanosheets
[0028] In a typical procedure, CoCl 2 ·6H 2 O, NaCl and hexamethylenetetramine (HMT) were sequentially dissolved in 200 mL of deionized water and ethanol at a volume ratio of 9:1 to obtain final concentrations of 10, 50 and 60 mM, respectively. Then the reaction solution was stirred and heated to 90° C. in an oil bath. After heating for about 1 h, a suspension containing green particles resulted. The solid product was collected by centrifugation at 7000 rpm for 2 min, and washed several times with deionized water and absolute ethanol. The final product was air dried at room temperature.
[0029] (2) Ag / α-Co(OH) 2 Synthesis of Nanocomposites
[0030] 50 mg of the above-prepared α-Co(OH) 2 The sample was dispersed in deionized water (40 mL) and ultrasonically dispersed for 5 min. Aqueous silver nitrate (79.5 μL, 10 mg mL -1 ) was configured into 1mL of Ag(NH 3 ) 2 OH solution, and then prepared 1mL of Ag(NH 3 ) 2 OH so...
Embodiment 2
[0039] Ag / α-Co(OH) with different Ag loading content 2 The preparation method of catalyst is substantially the same as that of Example 1, except that silver nitrate aqueous solution (238.5 μ L, 10 mg mL -1 ) was configured into 1 mL of Ag(NH 3 ) 2 OH solution, and the rest of the steps are the same; further characterization analysis and performance testing have been carried out to it, and embodiment 2 can achieve the purpose of the invention.
Embodiment 3
[0041] Ag / α-Co(OH) with different Ag loading content 2 The preparation method of catalyst is substantially the same as that of Example 1, except that silver nitrate aqueous solution (397.5 μ L, 10 mg mL -1 ) was configured into 1 mL of Ag(NH 3 ) 2 OH solution, the rest of the steps are the same; further character analysis and performance testing are carried out, and embodiment 3 can achieve the purpose of the invention.
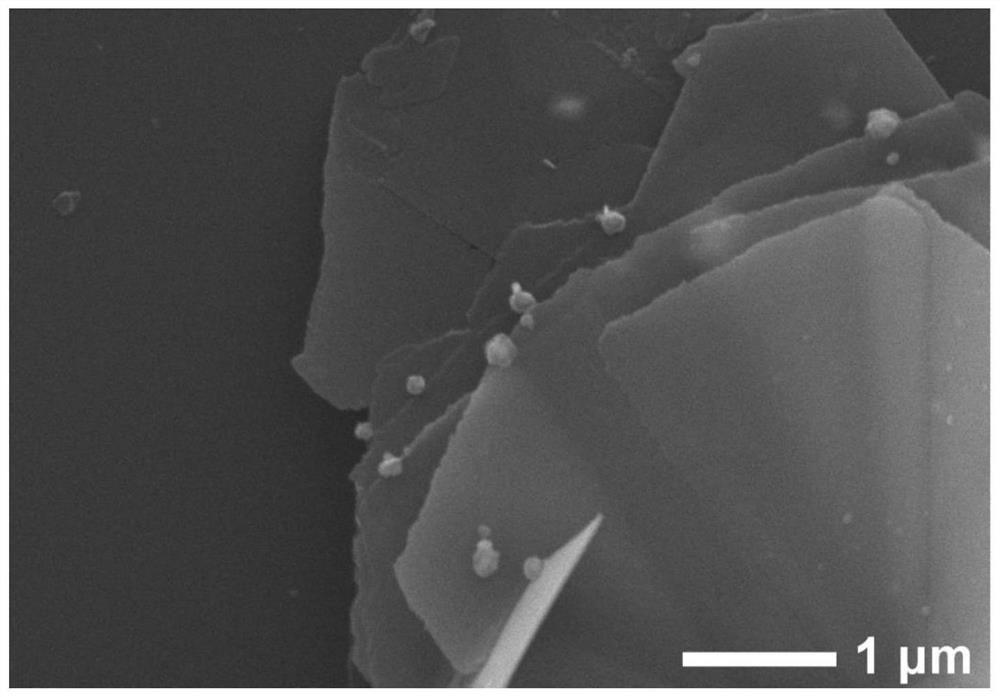
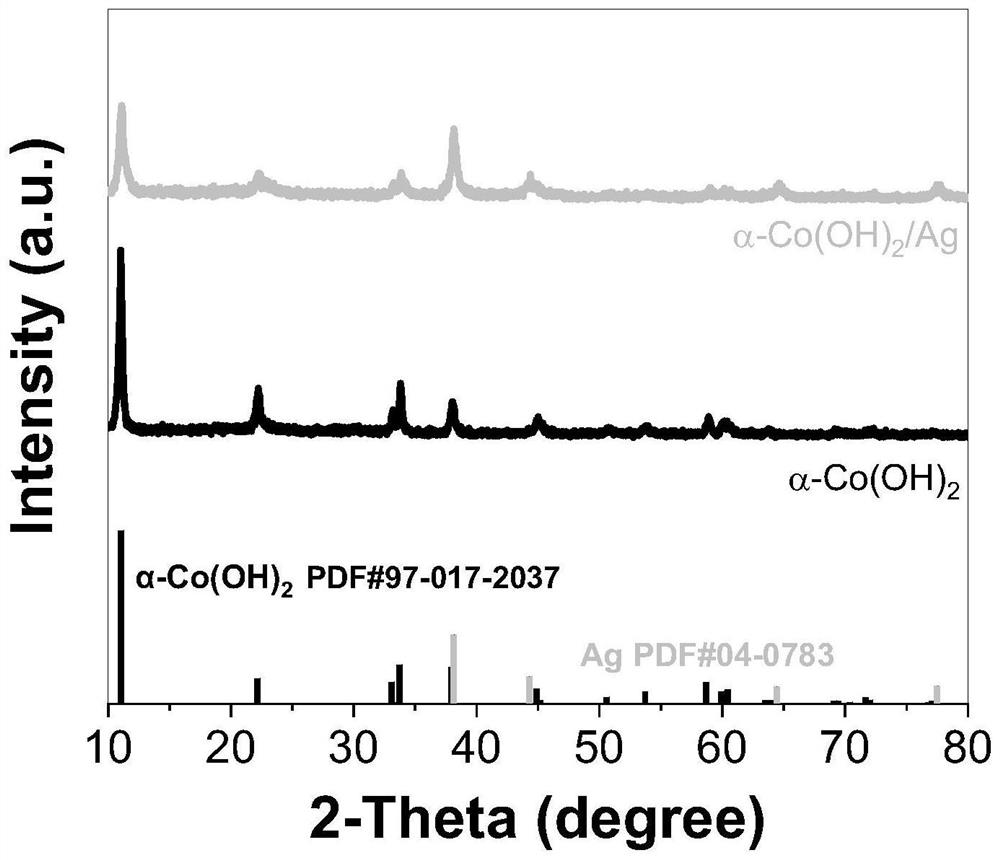
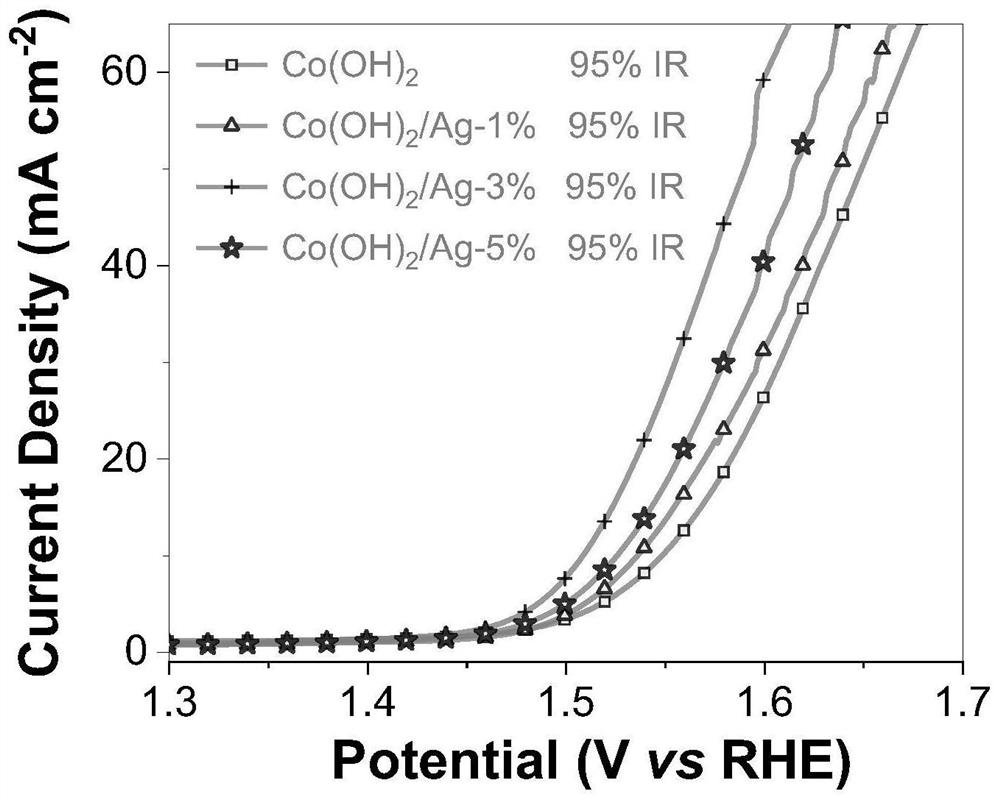
[0042] Test results: Ag / α-Co(OH) with different Ag loading contents were prepared by changing the concentration of added silver ammonia solution 2 The catalyst was subjected to X-ray diffraction and scanning electron microscopy results indicating the successful loading of Ag nanoclusters. A linear voltammetry sweep test was performed on it, such as image 3 Shown, embodiment 2 shows that when the current density is 10mA / cm -2 , the prepared Ag-supported Ag / α-Co(OH) 2 The overpotential of catalyst is reduced to 278mV, embodiment 3 shows that when current...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com