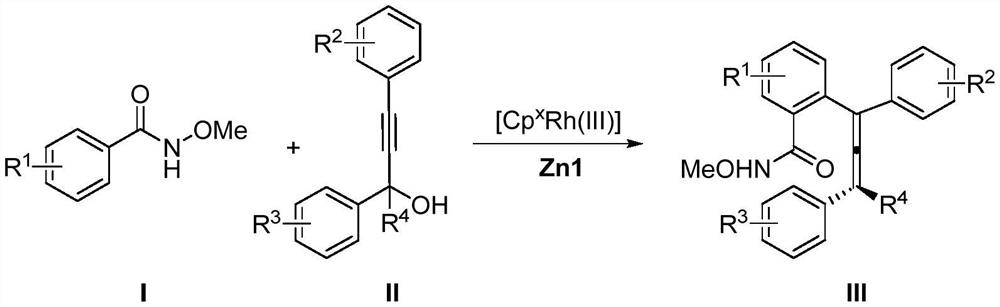
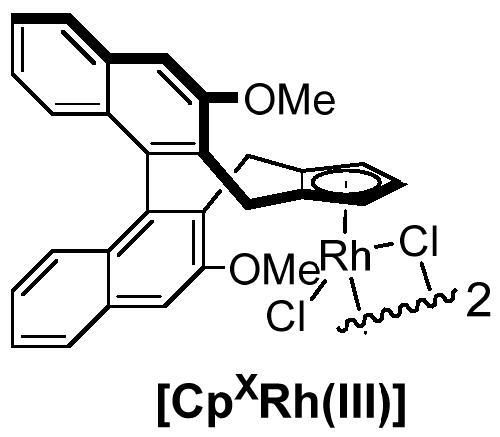
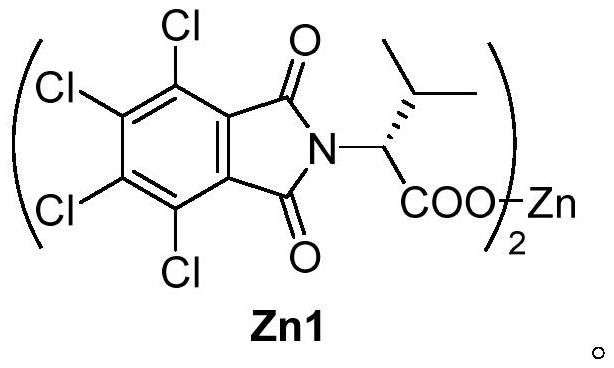
Method for synthesizing axially chiral allene compound under catalysis of trivalent rhodium
A compound and axial chirality technology, which is applied in the field of trivalent rhodium catalyzed synthesis of axial chiral allene compounds, can solve the problems of uncommon and limited biaryl compounds, and achieves easy preparation, good enantioselectivity and reaction conditions. mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Synthesis of 2-(1,2-dien-1-yl)-N-methoxybenzamide skeleton compound with the following structural formula
[0031]
[0032] Under air atmosphere, add 16.6mg (0.11mmol) N-methoxybenzamide, 52.9mg (0.2mmol) 4,4-dimethyl-1,3-diphenylpenta-1 -Alkyn-3-ol, 4.6mg (0.004mmol) [Cp X Rh(III)], 41.7mg (0.05mmol) chiral zinc carboxylate Zn1, 3mL 1,2-dichloroethane, tighten the pressure tube, stir and react at room temperature for 24 hours to make N-methoxybenzamide basically react completely , after the reaction was completed, 1,2-dichloroethane was removed by rotary evaporation under reduced pressure to obtain a crude product, which was passed through a column with silica gel (petroleum ether:ethyl acetate=10:1~3:1) to obtain a white solid Product, its yield is 54% (yield is calculated based on propargyl alcohol), and characterization data is: 1 H NMR (600MHz, CDCl 3 )δ8.34(s,1H),7.86(d,J=9.1Hz,1H),7.53(t,J=7.4Hz,1H),7.46(t,J=7.5Hz,1H),7.38–7.34( m,3H),7.32–7.28(m,5H),7.23–...
Embodiment 2
[0034] Synthesis of 2-(1,2-dien-1-yl)-N-methoxybenzamide skeleton compound with the following structural formula
[0035]
[0036] In this example, the N-methoxybenzamide used in Example 1 was replaced with equimolar 4-methyl-N-methoxybenzamide, and the other steps were the same as in Example 1 to obtain a white solid, Its yield is 38%, and characterization data is:1 H NMR (600MHz, CDCl 3 )δ8.29(s,1H),7.72(d,J=7.9Hz,1H),7.31–7.29(m,2H),7.25–7.24(m,2H),7.23–7.20(m,4H),7.16 –7.15(m,2H),7.14–7.13(d,J=7.3Hz,2H),7.11(s,1H),2.96(s,3H),2.36(s,3H),1.22(s,9H); 13 C NMR (151MHz, CDCl 3 )δ201.3, 165.7, 142.0, 136.5, 136.4, 134.5, 131.7, 130.4, 129.6, 129.3, 129.1, 129., 128.4, 127.6, 127.4, 126.2, 120.4, 108.3, 63.5, 36.1, 29.8, 2 Theoretical value C 28 h 29 NNaO 2 + [M+Na] + : 434.2091, measured value: 434.2085; HPLC: chiral column AD-H (n-hexane:isopropanol=95:5, 1.0mL / min, 40 ℃, 254nm); tr(major)=9.0min, tr(minor )=7.0min, 90%ee.
Embodiment 3
[0038] Synthesis of 2-(1,2-dien-1-yl)-N-methoxybenzamide skeleton compound with the following structural formula
[0039]
[0040] In this embodiment, the N-methoxybenzamide used in Example 1 is replaced with equimolar 4-phenyl-N-methoxybenzamide, and other steps are the same as in Example 1 (the reaction time is 30h ), obtain white solid product, and its yield is 50%, and characteristic data is: 1 H NMR (600MHz, CDCl 3 )δ8.5(s,1H),7.97(d,J=8.1Hz,1H),7.72(dd,J=8.1,1.8Hz,1H),7.66–7.61(m,3H),7.49–7.47(m ,2H),7.43–7.37(m,2H),7.36–7.30(m,6H),7.29–7.27(m,2H),7.21(t,J=7.2Hz,1H),3.06(s,3H), 1.30(s,9H); 13 C NMR (151MHz, CDCl 3 )δ201.5,165.4,144.3,139.7,136.4,136.3,135.2,131.1,130.9,129.7,129.3,129.1,129.0,128.4,128.3,127.6,127.5,127.2,126.9,126.3,120.6,108.3,63.6,36.1,29.8 ; HRMS (ESI) calculated for C 33 h 31 NNaO 2 + [M+Na] + :496.2247, found: 496.2244; HPLC: chiral column AD-H (n-hexane:isopropanol=95:5, 1.0mL / min, 40°C, 254nm); tr(major)=9.9min, tr(minor) =7.9min, 9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com