Polymer modified beta-glucosidase, preparation thereof and application of polymer modified beta-glucosidase in lignocellulose enzymolysis
A technology of glucosidase and polymer, applied in the direction of glycosylase, biochemical equipment and methods, enzymes, etc., can solve the problems of unfavorable cost, high energy consumption, inactivation of cellulase, etc., and achieve the change of surface properties , the effect of increasing affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] (1) The synthetic method of polymer modified β-glucosidase bgl-P (AM-b-AA-b-AN):
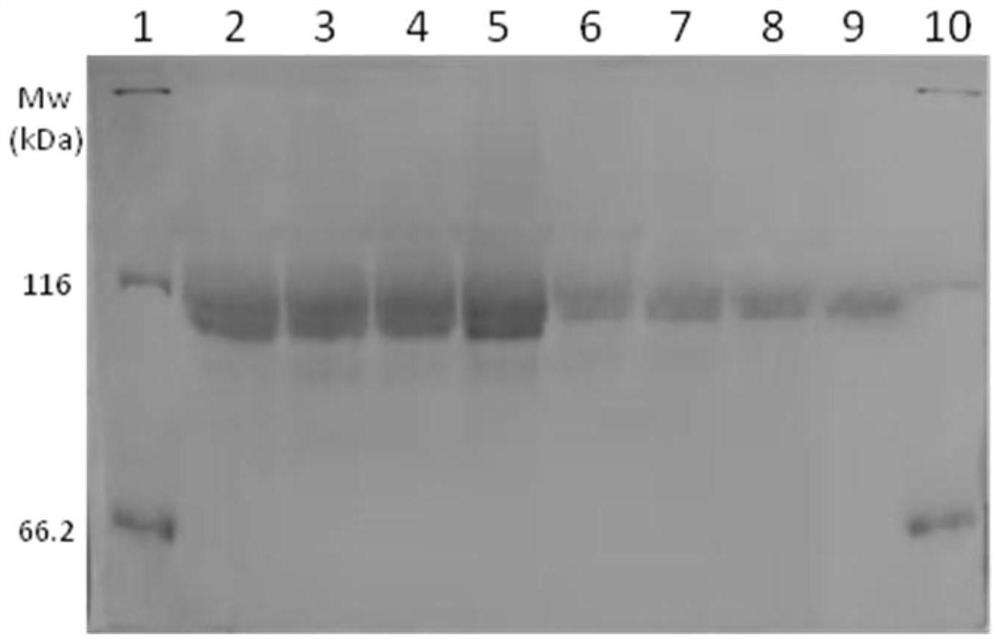
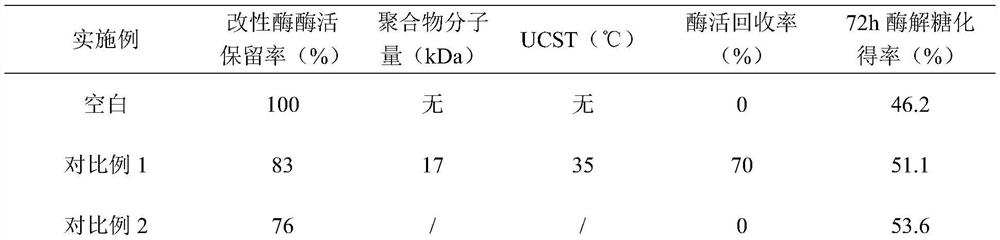
[0052] Dissolve 5g of acrylamide (AM) and 0.04g of azobisisobutyronitrile with 30ml of ethanol, transfer to a three-necked flask, deoxygenate with nitrogen, add 0.15g of chain transfer agent bis(carboxymethyl)trithiocarbonate, The reaction was stirred at 80°C for 8h, and 1.28g of acrylic acid (AA) and 1g of acrylonitrile (AN) were added dropwise to continue the reaction for 6h. Cool and centrifuge, take the precipitate and add methanol to heat up and resuspend, repeat three times, and vacuum dry to obtain a gel-like product. Add 45 mg of the above product into 20 ml of 10 mg / ml β-glucosidase phosphate buffer, adjust the pH to 8.0, add 0.45 g of EDC and 0.3 g of NHS in sequence, and react with magnetic stirring at 50 ° C for 2 h. The product was purified and concentrated by 100kDa membrane ultrafiltration, adjusted to pH 4.0, stored at 4°C, and tested for its enzyme activity and phase tra...
Embodiment 2
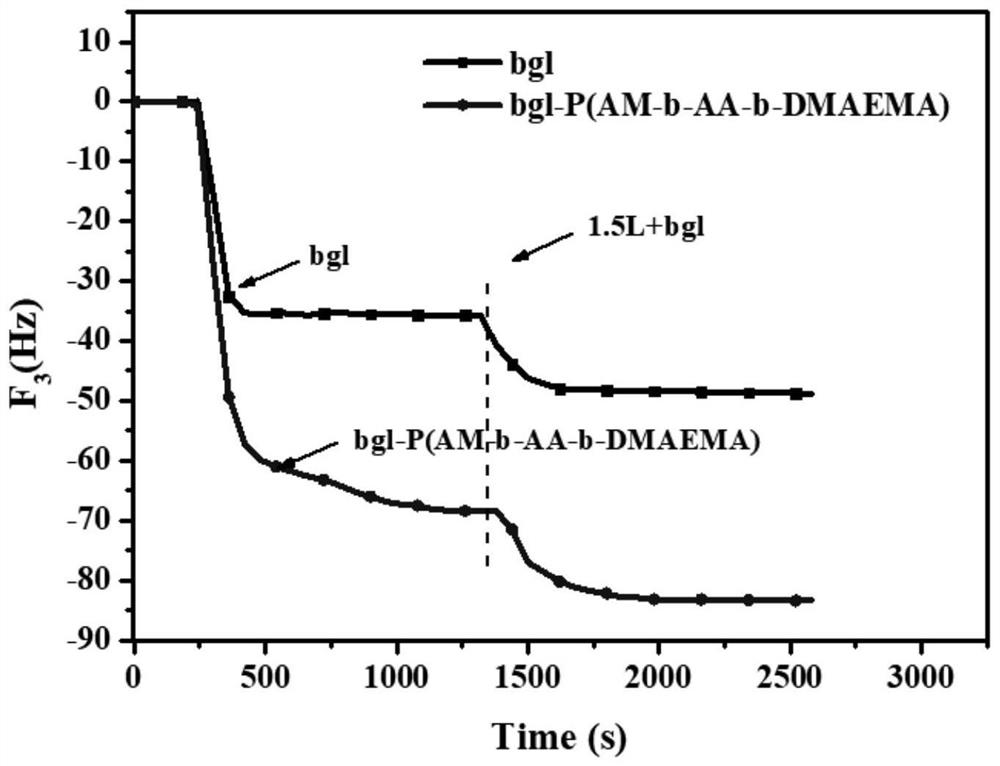
[0056] (1) The synthetic method of modified β-glucosidase bgl-P (AM-b-AA-b-DMAEMA):
[0057] Dissolve 4.95g of acrylamide and 0.022g of ammonium persulfate in 30ml of pure water, transfer to a three-necked flask, deoxygenate with nitrogen, add 0.08g of chain transfer agent mercaptopropionic acid, stir and react at 80°C for 8h, add dropwise 1.28g of acrylic acid (AA ) and 7.8g dimethylaminoethyl methacrylate (DMAEMA) continued to react for 5h. Cool and centrifuge, take the precipitate and add pure water to raise the temperature and resuspend, repeat three times, and vacuum dry to obtain a gelatinous product. Take 30 mg of the above product and add it into 10 ml of 10 mg / ml β-glucosidase phosphate buffer solution, adjust the pH to 8.0, followed by 0.45 g of EDC and 0.3 g of NHS, and react with magnetic stirring at 40 ° C for 2 h. The product was purified and concentrated by 100kDa membrane ultrafiltration, adjusted to pH 4.0, stored at 4°C, and tested for its enzyme activity an...
Embodiment 3
[0061] (1) The synthetic method of polymer modified β-glucosidase bgl-P (AM-b-AA-b-st):
[0062] Dissolve 4.95g of acrylamide (AM) and 0.02g of azobisisobutyronitrile in 10ml of dimethyl sulfoxide, transfer to a three-necked flask, deoxygenate with nitrogen, add 0.15g of chain transfer agent 2-(dodecyltri Thiocarbonate group)-2-methylpropionic acid, stirred at 70°C for 8h, added dropwise 1.28g of acrylic acid and 1.05g of styrene (st) to continue the reaction for 20h. Cool and centrifuge, take the precipitate and add methanol to heat up and resuspend, repeat three times, and vacuum dry to obtain a gel-like product. Add 45 mg of the above product into 10 ml of 10 mg / ml β-glucosidase phosphate buffer, adjust the pH to 8.0, add 0.45 g of EDC and 0.3 g of NHS in sequence, and react with magnetic stirring at 50 ° C for 2 h. The product was purified and concentrated by 100kDa membrane ultrafiltration, adjusted to pH 4.0, stored at 4°C, and tested for its enzyme activity and phase t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com