V-shaped chiral carboxylic acid ligand, preparation method and application thereof
A chiral carboxylic acid and ligand technology, applied in organic chemistry methods, organic chemistry, etc., can solve the problem of variable coordination modes, difficulty in obtaining asymmetric catalytic and separation functional chiral complex materials, and synthesis of chiral ligands high cost issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
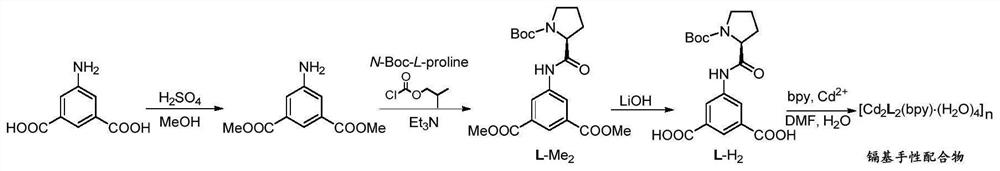
[0042] V-case-chiral carboxylic acid ligand L-H 2 Preparation method, synthetic route schematic figure 1 As shown, specifically includes the following steps:
[0043]1) The V-shaped precursor 5-amino benzene terephthalic acid (5.0 g, 27.6 mmol) was dissolved in 150 ml of methanol, slowly dropped concentrated sulfuric acid (3.0 ml, 17.0 mmol), and the reaction mixture was refluxed for 12 hours, then stopped the reaction. It was cooled to room temperature; then a small amount of water was added to dilute the concentrated sulfuric acid, evaporated, then the pH to 7 of the reaction mixture was adjusted, and the precipitated white solid was filtered, washed, dried to give an intermediate product 5- Aminomethylene terephthalate 5.37 g, yield 93%.
[0044] 2) L-BOC-proline (3.55 g, 16.5 mmol) as a chiral source is dissolved in 100 mL of dichloromethane, and isochloromethoate (2.85 mL, 20.0 mmol), three, respectively, and three, respectively. Ethylamine (2.8 mL, 20.0 mmol) and 5-aminoethy...
Embodiment 2
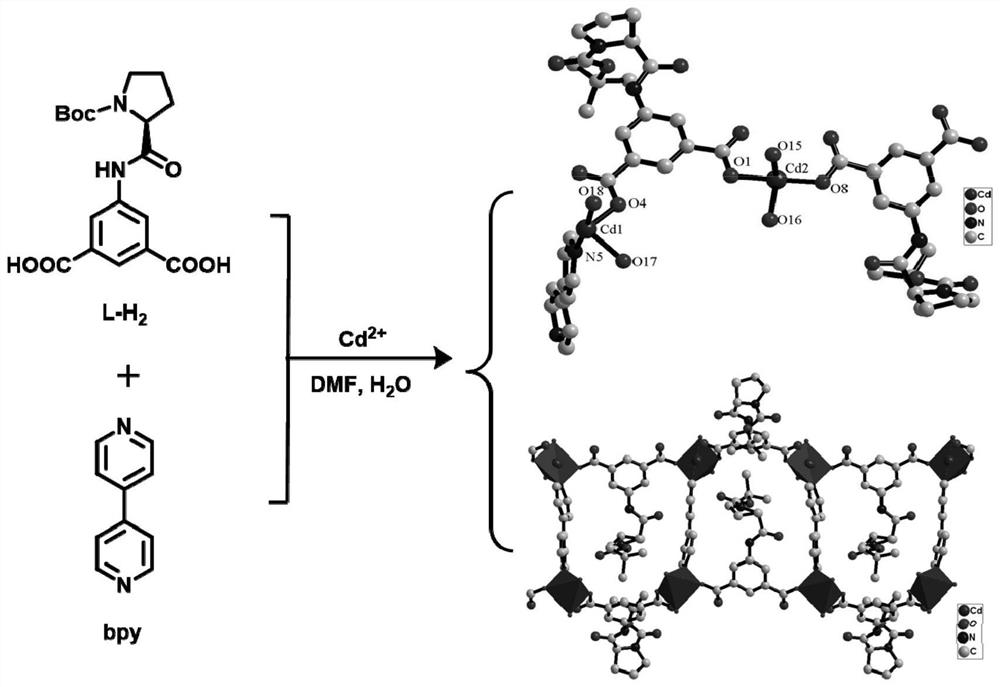
[0047] Preparation method of cadmium-based chiral complex, synthetic route schematic image 3 As shown, specifically includes the following steps:
[0048] CD (OAC) weighing 2 · 2h 2 O (5.33mg, 0.02mmol), L-H 2 (8.3 mg, 0.02 mmol), 4,4'-bond pyridine (BPY) (1.56 mg, 0.01 mmol) is dissolved in DMF (0.5 mL) and H 2 O (0.5 mL) mixed solvent, and sealed in explosion-proof glass, 60 ° C heating for 48 hours, cooled to obtain a colorless transparent block crystal, then filtered, ethanol, natural air dry, resulting 6.4 mg cadmium base hand Sexual complex, repeatedly re-responded, and the resulting large number of chiral complexs were measured by powder X-ray diffraction (PXRD), and the results showed the PXRD spectrum of the experiment and the PXRD spectra of single crystal structure simulated, proved. Pure phase purity is better ( Figure 4 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com