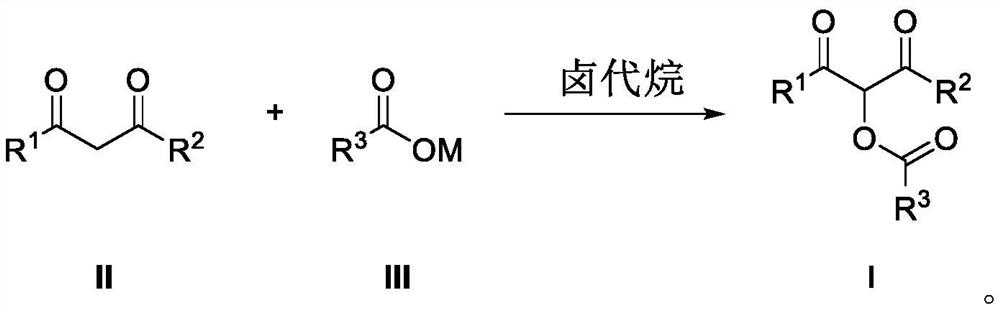
Preparation method of alpha-acyloxy ketone compound
A technology for acyloxy ketones and dicarbonyl compounds is applied in the field of preparation of α-acyloxy ketone compounds, and can solve the problems of harsh reaction conditions, expensive acyloxylation reagents, complicated operation and the like, and achieves mild reaction conditions, Good industrial application prospects, simple construction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
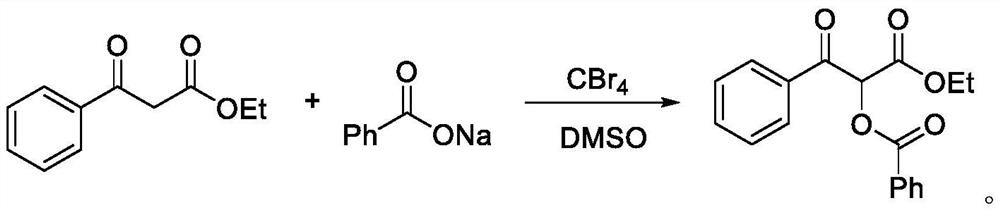
[0021] The embodiment of the present invention provides a preparation method of α-acyloxyketone compound, specifically, take a dry reaction tube, add 0.4mmol ethyl benzoylacetate, 0.4mmol sodium benzoate, 2mL dimethyl sulfoxide and 0.4mmol carbon tetrabromide, stirred and reacted at a temperature of 20-30°C for 0.5h to obtain a mixture; then 5mL of water was added to the mixture to quench, and extracted three times with 10mL of ethyl acetate, so that the organic phase Separate from the water phase, combine the extracts, wash the organic phase with saturated brine, and dry with anhydrous sodium sulfate; then use a rotary evaporator to remove the organic solvent to obtain a crude product of α-acyloxyketone compound, and use a 200-300-mesh Silica gel was separated by column chromatography, and the eluent was prepared from petroleum ether and ethyl acetate at a volume ratio of 10:1. The crude product was separated by column chromatography to obtain a pure α-acyloxyketone compound w...
Embodiment 2
[0029] The embodiment of the present invention provides a preparation method of α-acyloxyketone compound, specifically, take a dry reaction tube, add 0.4mmol dibenzoylmethane, 0.4mmol sodium benzoate, 2mL dimethylmethylene Sulfone and 0.4mmol N-bromosuccinimide were stirred and reacted at a temperature of 20 to 30°C for 0.5h to obtain a mixture, then 5mL of water was added to the mixture to quench, and extracted three times with 10mL of ethyl acetate, thereby The organic phase and the aqueous phase were separated, the extracts were combined, the organic phase was washed with saturated brine, and dried with anhydrous sodium sulfate; ~300 mesh silica gel was separated by column chromatography. The eluent was prepared from petroleum ether and ethyl acetate at a volume ratio of 10:1. The crude product was separated by column chromatography to obtain a pure compound of α-acyloxyketone. The yield 88%. Its reaction formula is as follows:
[0030]
[0031] The reaction product th...
Embodiment 3
[0037] The embodiment of the present invention provides a preparation method of α-acyloxyketone compound, specifically, take a dry reaction tube, add 0.4mmol N-acetylacetonate morpholine, 0.4mmol sodium benzoate, 2mL N,N - Dimethylformamide and 0.4mmol carbon tetrabromide, stirred and reacted at a temperature of 20 to 30°C for 0.5h to obtain a mixture, then quenched by adding 5mL of water to the mixture, and extracted three times with 10mL of ethyl acetate, Thereby, the organic phase and the aqueous phase are separated, the extracts are combined, the organic phase is washed with saturated brine, and dried with anhydrous sodium sulfate; 200-300 mesh silica gel was separated by column chromatography. The eluent was prepared from petroleum ether and ethyl acetate at a volume ratio of 10:1. The crude product was separated by column chromatography to obtain a pure compound of α-acyloxyketone. rate of 77%. Its reaction formula is as follows:
[0038]
[0039] The reaction produ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com