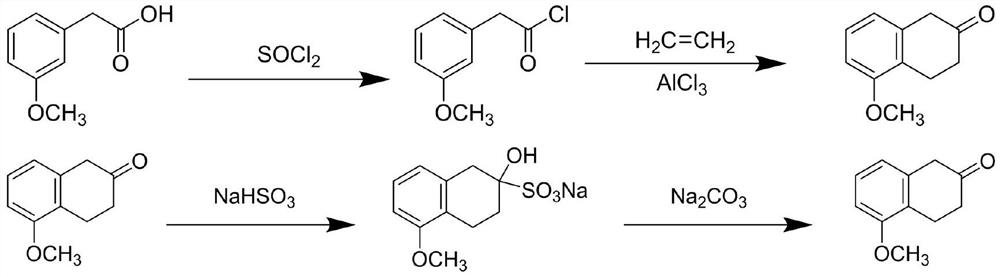
Synthesis method of 5-methoxy-2-tetralone
A synthetic method and technology of tetralone, which is applied in the field of synthesis of 5-methoxy-2-tetralone, can solve the problems that the use of sodium is easily affected by environmental humidity, unfavorable for safe production, and expensive synthetic raw materials, etc. Short steps, easy operation, less three waste pollutants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Add 1.66g (10mmol) 3-methoxyphenylacetic acid and magneton into a 50mL three-necked flask, install a condenser tube and a dropping funnel, place the instrument in an oil bath, set the temperature to 55°C, and turn on the stirring and condenser tube , then add 10mL of thionyl chloride and 0.5mL of N,N-dimethylformamide into the dropping funnel, wait until the temperature rises to 55°C, start the dropwise addition, and slowly add the solution into the reactor at a rate of 0.1mL / s, after the dropwise addition is completed, reset the heating temperature to 90°C, stir for 3 hours, and use TLC to confirm that the reaction is complete and prepare to start purification.
[0034] After the liquid in the reactor was cooled to room temperature, it was taken out in an eggplant-shaped bottle, and the contents of the eggplant-shaped bottle were rotated under reduced pressure at 60°C to remove excess thionyl chloride. After the rotary evaporation was completed, 30 mL of n-hexane was a...
Embodiment 2
[0039] Add 1.66g (10mmol) 3-methoxyphenylacetic acid and magneton into a 50mL three-necked flask, install a condenser tube and a dropping funnel, place the instrument in an oil bath, set the temperature to 55°C, and turn on the stirring and condenser tube , and then add 10mL of thionyl chloride and 0.5mL of N,N-dimethylformamide into the dropping funnel, wait until the temperature rises to 55°C, start the dropwise addition, and slowly add the solution into the reactor at a rate of 0.1 mL / s, after the dropwise addition, reset the heating temperature to 80°C, stir the reaction for 3 hours, use TLC to confirm that the reaction is complete, and prepare to start purification.
[0040] After the liquid in the reactor was cooled to room temperature, it was taken out in an eggplant-shaped bottle, and the contents of the eggplant-shaped bottle were rotated under reduced pressure at 60°C to remove excess thionyl chloride. After the rotary evaporation was completed, 30 mL of n-hexane was ...
Embodiment 3
[0045] Add 1.66g (10mmol) 3-methoxyphenylacetic acid and magneton into a 50mL three-necked flask, install a condenser tube and a dropping funnel, place the instrument in an oil bath, set the temperature to 55°C, and turn on the stirring and condenser tube , and then add 10mL of thionyl chloride and 0.5mL of N,N-dimethylformamide into the dropping funnel, wait until the temperature rises to 55°C, start the dropwise addition, and slowly add the solution into the reactor at a rate of 0.1 mL / s, after the dropwise addition, reset the heating temperature to 85°C, stir the reaction for 3 hours, use TLC to confirm that the reaction is complete, and prepare to start purification.
[0046] After the liquid in the reactor was cooled to room temperature, it was taken out in an eggplant-shaped bottle, and the contents of the eggplant-shaped bottle were rotated under reduced pressure at 60°C to remove excess thionyl chloride. After the rotary evaporation was completed, 30 mL of n-hexane was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com