Synthesis method of bismaleimide containing adamantane side group triarylamine
A bismaleimide and synthesis method technology, applied in organic chemistry and other fields, can solve problems such as steric regularity and polymer solubility constraints, and achieve high free volume, improved solubility, and excellent benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
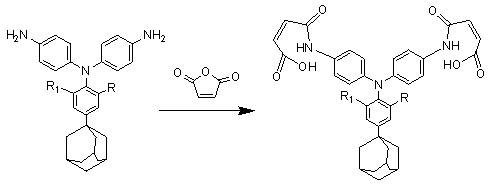
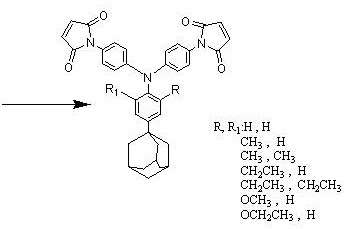
[0024] The synthetic preparation method of the triarylamine bismaleimide containing adamantane side group mainly comprises following route:
[0025] Synthesis of triarylamine diamine containing adamantane side group:
[0026]
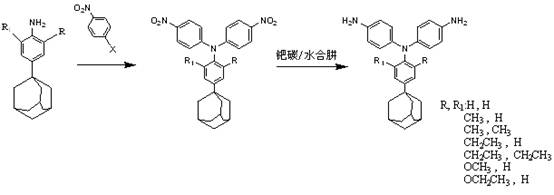
[0027] p-adamantylaniline and p-halogenated nitrobenzene, in which the halogen atom can be chlorine, bromine, fluorine, preferably fluorine atom, dissolved in dimethyl sulfoxide according to the molar ratio of 1:2~2.1, at 110°C-150 Reaction at ℃ for 12-16h, using cesium fluoride as a catalyst, the dosage is 50%-90% of the p-halogenated nitrobenzene substance, after the reaction, pour the mother liquor into water to precipitate, then suction filter, wash and dry Obtain dinitro compounds, and then use acetonitrile to carry out recrystallization;
[0028] The palladium-carbon catalytic reduction system is selected for the dinitro compound, and the triarylamine diamine compound is obtained under the action of hydrazine hydrate, and finally recrystallize...
Embodiment 1
[0036] Take 0.1 mol of p-adamantylaniline and 0.21 mol of p-fluoronitrobenzene, dissolve them in a three-neck flask with a thermometer and a magnetic rotor containing 100 ml of dimethyl sulfoxide, and add 0.16 mol of fluorine after the dissolution is complete Cesium chloride was heated to 120°C for 15 hours. After the reaction was complete, the solution was poured into water, stirred rapidly, settled for 12 hours, filtered and washed several times with suction, and dried to obtain a yellow solid crude product. The crude dinitro compound was recrystallized from acetonitrile.
[0037] Take 0.1 mol of the recrystallized dinitro compound, add it to a three-necked flask with a thermometer and a magnetic rotor containing 100 ml of ethanol, add 10% palladium carbon based on the mass of the dinitro compound, stir evenly, and slowly Hydrazine hydrate was added to raise the temperature to reflux temperature, and the reaction was carried out for 12 hours. After the reaction is complete...
Embodiment 2
[0040] Take 0.5 mol of p-adamantylaniline and 1.1 mol of p-chloronitrobenzene and dissolve them in a three-neck flask containing 500 ml of dimethyl sulfoxide with a thermometer and a magnetic rotor. After the dissolution is complete, add 0.8 mol of fluorinated cesium, heated to 150 ° C for 15 hours. After the reaction was complete, the solution was poured into water, stirred rapidly, settled for 12 hours, filtered and washed several times with suction, and dried to obtain a yellow solid crude product. The crude dinitro compound was recrystallized from acetonitrile.
[0041]Take 0.4 mol of the recrystallized dinitro compound and add it to a three-neck flask with a thermometer and a magnetic rotor containing 350 ml of ethanol. Based on the mass of the dinitro compound, add 5% palladium carbon, stir evenly, and slowly add Hydrazine hydrate was warmed up to reflux temperature and reacted for 12h. After the reaction is complete, remove the palladium carbon by suction filtration w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com