Alkali metal-rare earth dissimilar metal framework compound, and preparation method and application thereof
A framework compound and heterometallic technology, which is applied in the field of photoelectric functional materials and crystal materials, can solve the problems of battery electrochemical performance degradation, affecting battery life, and low lithium ion mobility, achieving high ion conductivity and lithium ion migration number effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
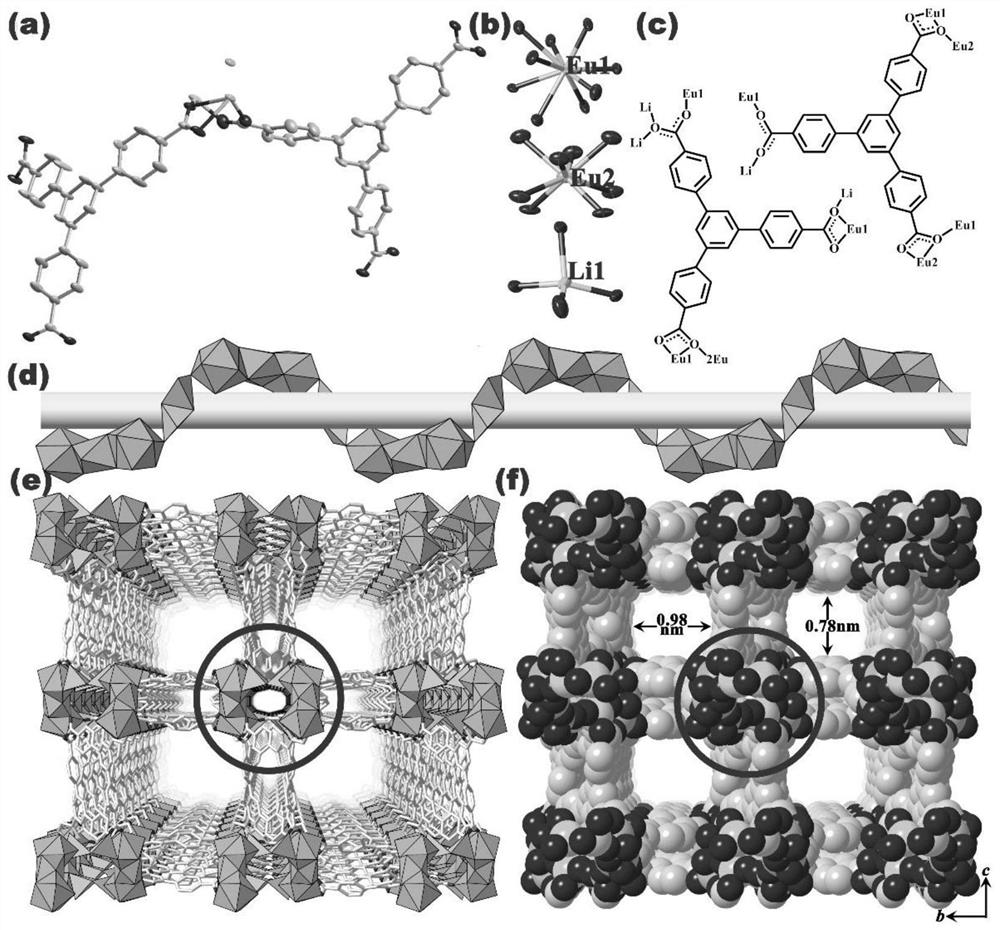
[0035] Example 1: [NH 2 (CH 3 ) 2 ][Eu 3 Li 2 (BTB) 4 ] Preparation of Heterometallic Organic Framework Compounds
[0036] Mix 0.135 mmol europium acetate hydrate, 0.1 mmol 1,3,5-tris(4-carboxyphenyl)benzene and 0.4 mmol lithium hydroxide monohydrate (or 0.1 mmol europium acetate hydrate, 0.12 mmol 1,3,5-tris( 4-carboxyphenyl)benzene and 0.4mmol lithium hydroxide monohydrate) were added to the mixed solution of 4mL N,N-dimethylacetamide and 1mL water and mixed evenly, the resulting mixture was put into a 25mL stainless steel reaction kettle, and put into the oven for hydrothermal reaction. After being incubated at 130°C for 72 hours, it was cooled to room temperature, filtered and centrifuged to obtain [NH 2 (CH 3 ) 2 ][Eu 3 Li 2 (BTB) 4 ] Heterometallic organic framework compounds.
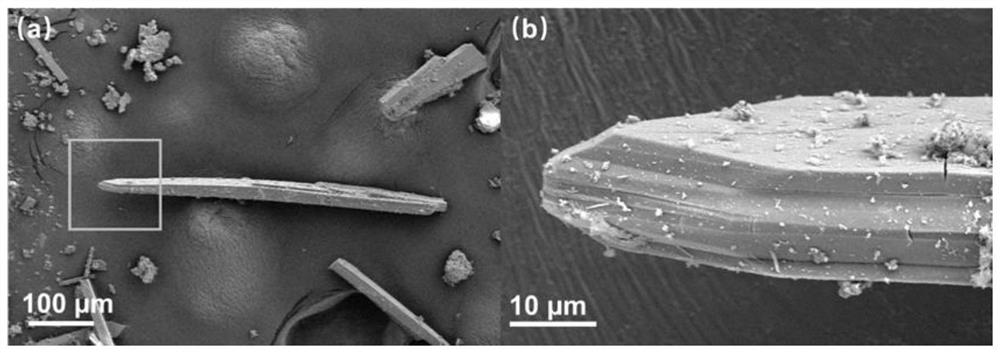
[0037] The resulting compound is subjected to a scanning electron microscope test, and its scanning electron microscope is as follows: figure 1 shown.
[0038] The samples were cha...
Embodiment 2
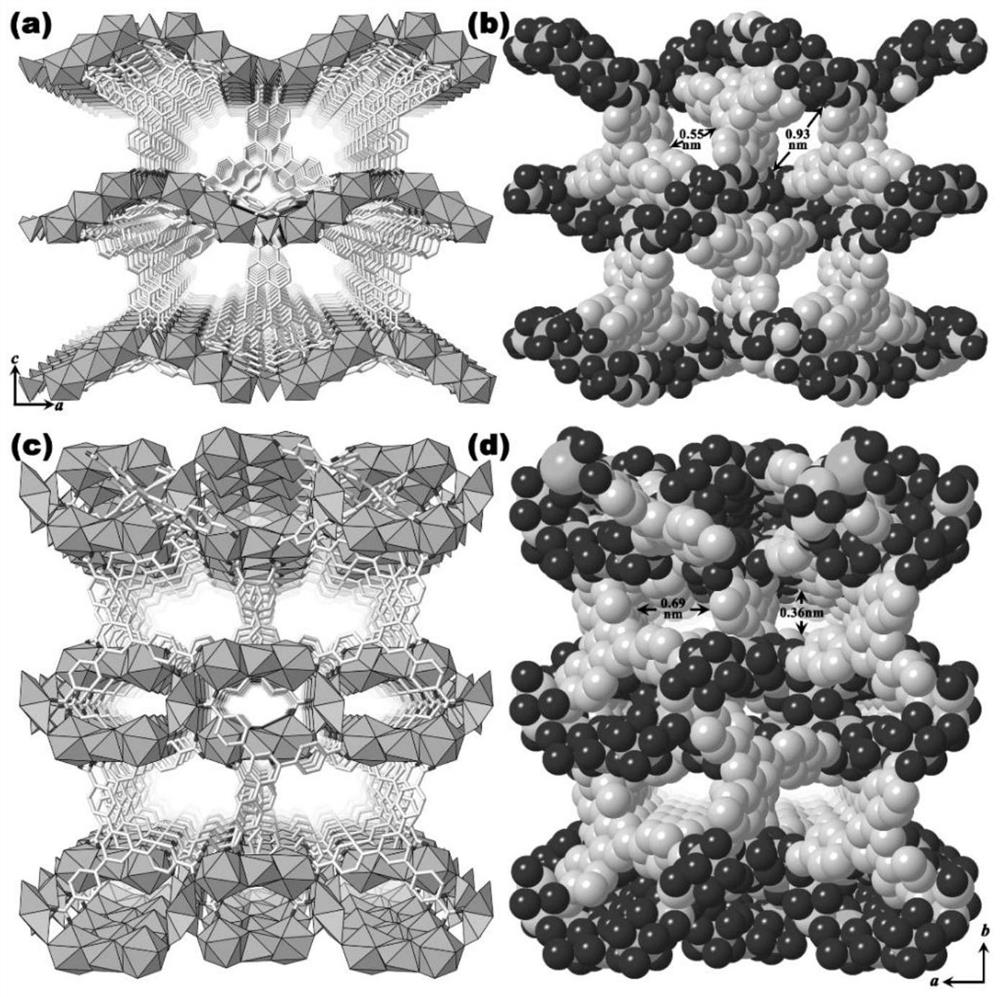
[0045] Example 2: [NH 2 (CH 3 ) 2 ][Eu 3 Li 2 (TATB) 4 ] Preparation of Heterometallic Organic Framework Compounds
[0046] Mix 0.135mmol europium acetate hydrate, 0.1mmol 2,4,6-tris(4-carboxyphenyl)-1,3,5-triazine and 0.4mmol lithium hydroxide monohydrate (or 0.1mmol europium acetate hydrate, 0.12mmol 2,4,6-tris(4-carboxyphenyl)-1,3,5-triazine and 0.4 mmol lithium hydroxide monohydrate) were added to 4 mL of N,N-dimethylacetamide, 1 mL of water, and 24 μL of tris The mixed solution of ethylamine was uniformly mixed, and the resulting mixture was put into a 25mL stainless steel reaction kettle, and put into an oven for hydrothermal reaction. After being incubated at 160°C for 36 hours, it was lowered to room temperature, filtered and centrifuged to obtain [NH 2 (CH 3 ) 2 ][Eu 3 Li 2 (TATB) 4 ] Heterometallic organic framework compounds.
[0047] The metal Eu in Example 2 can be completely replaced by Y, Ho, Dy, Er, Tb, Tm, etc. to form completely isomorphic compou...
Embodiment 3
[0048] Example 3: [NH 2 (CH 3 ) 2 ][Y 3 Li 2 (BTB) 4 ] Preparation of Heterometallic Organic Framework Compounds
[0049] Add 0.1 mmol of yttrium acetate hydrate, 0.12 mmol of 1,3,5-tris(4-carboxyphenyl)benzene and 0.4 mmol of lithium hydroxide monohydrate to a mixed solution of 4 mL of N,N-dimethylacetamide and 1 mL of water Mix evenly, put the resulting mixture into a 25mL stainless steel reaction kettle, and put it into an oven for hydrothermal reaction. After being incubated at 120°C for 84 hours, it was lowered to room temperature, filtered and centrifuged to obtain [NH 2 (CH 3 ) 2 ][Y 3 Li 2 (BTB) 4 ] Heterometallic organic framework compounds.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ionic conductivity | aaaaa | aaaaa |
| Ionic conductivity | aaaaa | aaaaa |
| Initial discharge specific capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com