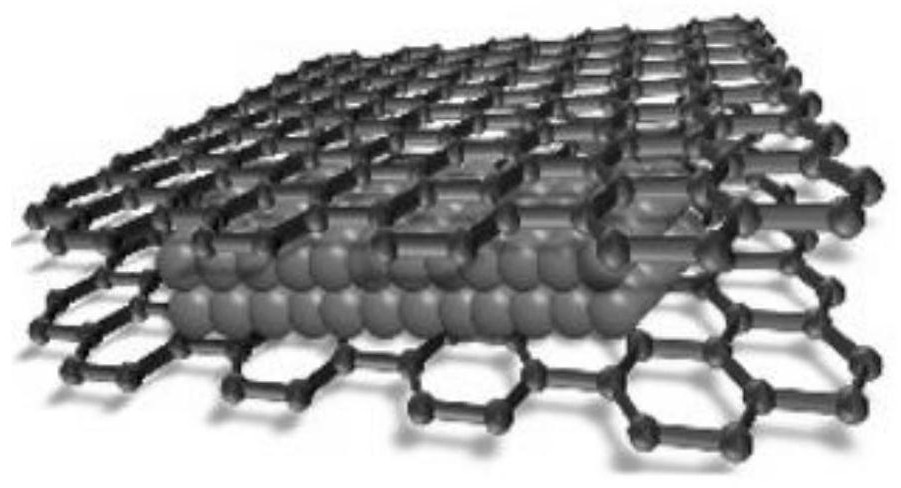
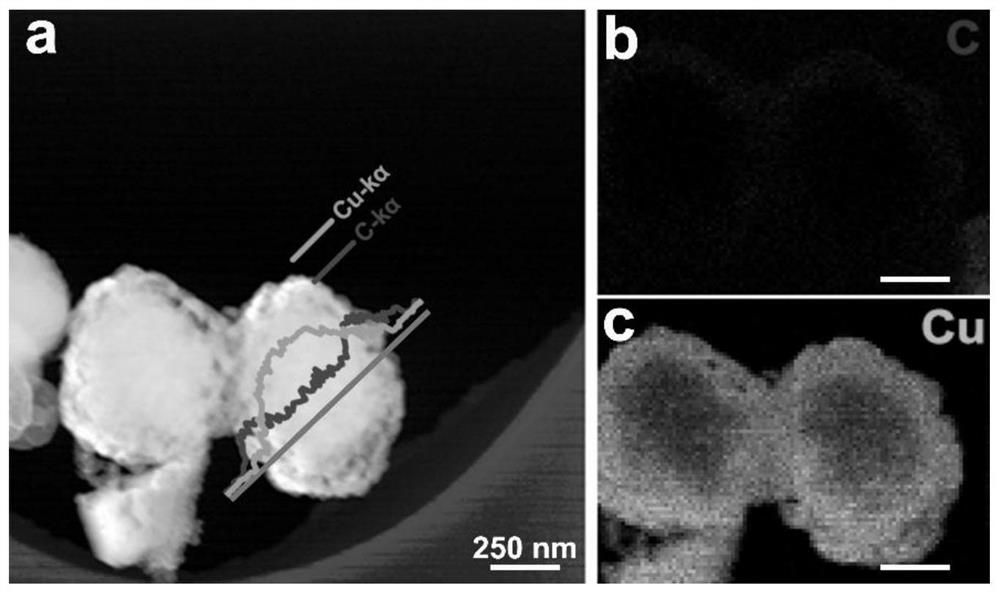
Carbon interlayer copper nanosheet electrocatalyst with sandwich structure, preparation method, electrode and application
An electrocatalyst and copper nanotechnology, applied in the field of electrocatalysis, can solve the problems of rapid deactivation of metal copper catalysts, and achieve the effects of improving reduction efficiency, preventing oxidation, and promoting rate-limiting steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] According to a second aspect of the present invention, a method for preparing a sandwich-structured carbon interlayer copper nanosheet electrocatalyst is provided, comprising the steps of:
[0035] Step 1, dissolve the soluble copper salt and the reducing agent in the reaction vessel with water, stir evenly, add cetyltrimethylammonium bromide (CTAB) and hexamethamine (HMTA), and react for at least 30 minutes. The container is sealed, and then from room temperature to 2 ~ 4 ℃ · min -1 The speed is raised to 80-90°C to continue the reaction, preferably at 80-90°C for 3-4 hours, then cooled to room temperature, and the obtained product copper nanosheets are dispersed in ethanol solution for subsequent use after centrifugal cleaning;
[0036] Step 2, pouring the prepared copper nanosheets into the tirs-HCl dopamine buffer solution, stirring to cause self-polymerization of dopamine on the copper nanosheets, after the reaction is completed, the product is centrifugally cleane...
Embodiment 1
[0050] Take 50mg of copper nitrate trihydrate (Cu(NO 3 ) 2 ·3H 2 0), 100 mg vitamin C and 15 mL deionized water were added to a 20 mL reaction vessel. It was uniformly mixed using magnetic stirring, and then 100 mg of cetyltrimethylammonium bromide (CTAB) and 100 mg of urotropine (HMTA) were added. After 30 minutes of reaction, the reaction vessel was sealed, and then from room temperature at 2°C min -1 The speed was raised to 80°C, and the reaction was carried out at 80°C for 3 hours. Finally, the room temperature was cooled, and the obtained product was washed by centrifugation, and dispersed in an ethanol solution for later use.
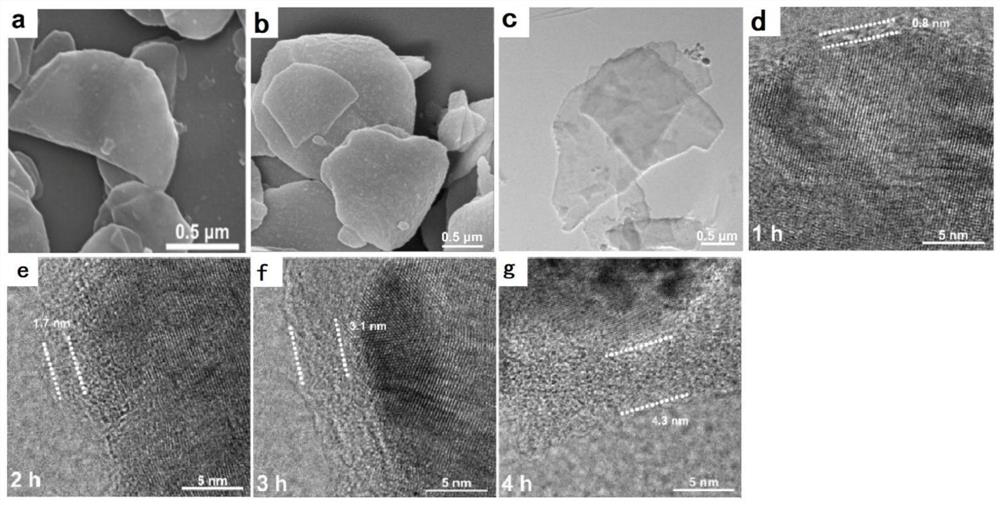
[0051] Pour the prepared copper nanosheets (20mg) into 30mL tirs-HCl dopamine (3mg·mL -1 ) in the buffer solution, the magnetic speed allows dopamine to self-polymerize through magnetic stirring (1500 rpm), and the reaction time is recorded as 1 hour. After self-polymerization, the product was centrifuged and cleaned, and then placed in an o...
Embodiment 2~4
[0053] Embodiments 2 to 4 are the same as Embodiment 1, except that the time for self-polymerization of dopamine is different, which are 2, 3 and 4 hours respectively.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com