Three-arm mannose derivative and preparation method thereof through combination with double-click chemistry
A click chemistry, mannose technology, applied in the preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., can solve the problems of preparation process obstacles, single sugar unit structure, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Synthesis of Compound B
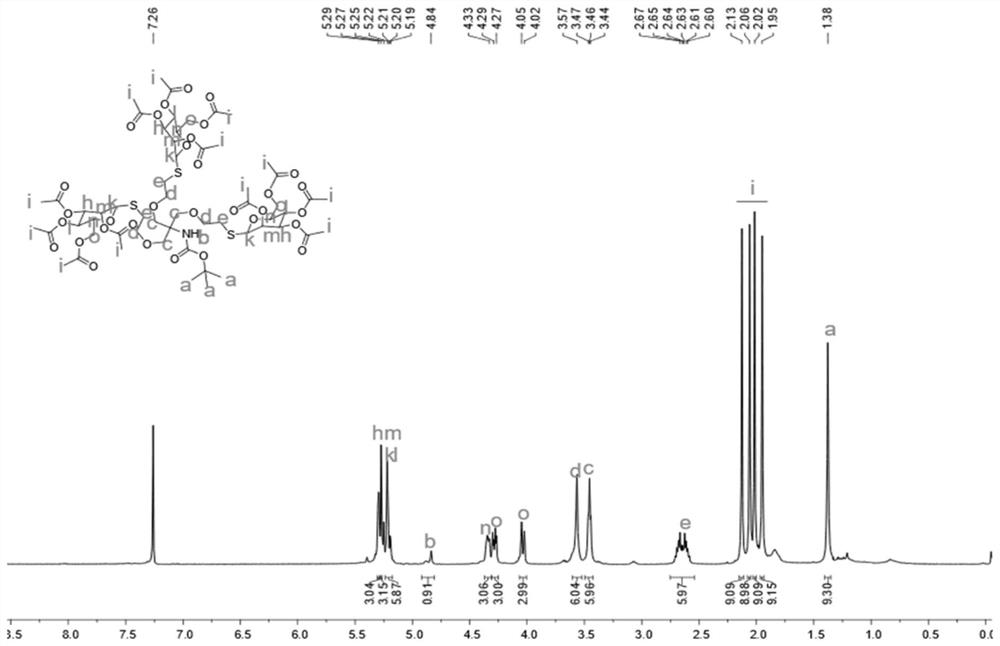
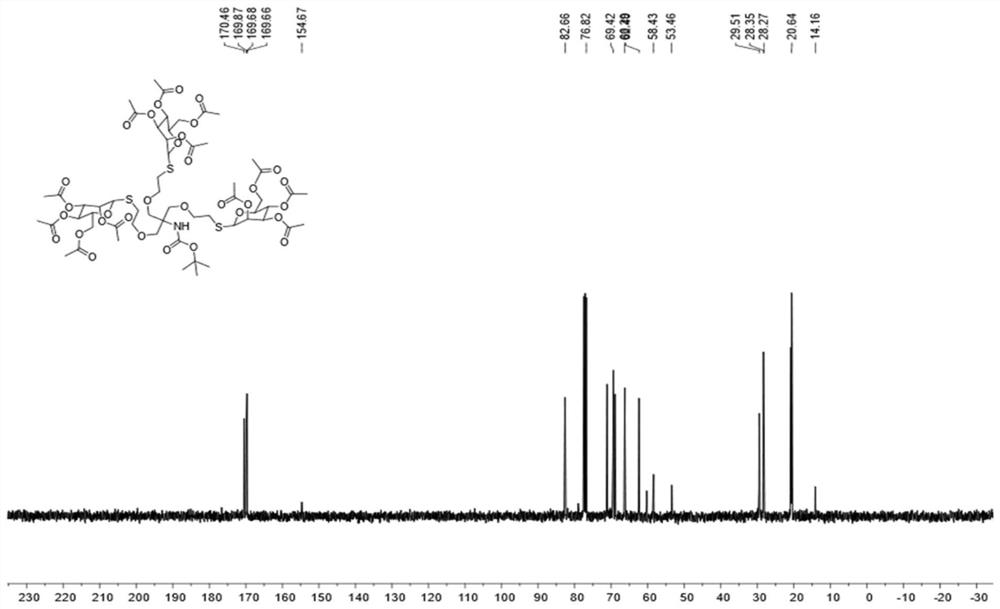
[0070] Compound A (1,3-bis(allyloxy)-2-((allyloxy)methyl)prop-2-yl) tert-butyl carbamate (0.3g, 0.8mmol) and mercaptomannose (α-Man-SH) (1.1g, 3.1mmol) was dissolved in 5mL of anhydrous dichloromethane solution, followed by the addition of 2,2-dimethoxy-2-acetophenone (DMPA) (22.5mg, 0.09mmol ). Irradiate with 365nm ultraviolet light at room temperature and stir for 45min. After TLC monitoring until the reaction is completed, the reaction solution was washed with dichloromethane (50mL×3) and saturated brine (50mL×3) respectively, and the organic phase collected after extraction was dried with anhydrous sodium sulfate, filtered, and distilled under reduced pressure After concentration and purification by column chromatography, 1.1 g of a light yellow viscous product was obtained, namely compound B, with a yield of 90%.
[0071] The H NMR spectrum and the C NMR spectrum of the obtained compound B can be found in figure 1 and figure 2 shown....
Embodiment 2
[0078] Synthesis of Compound D
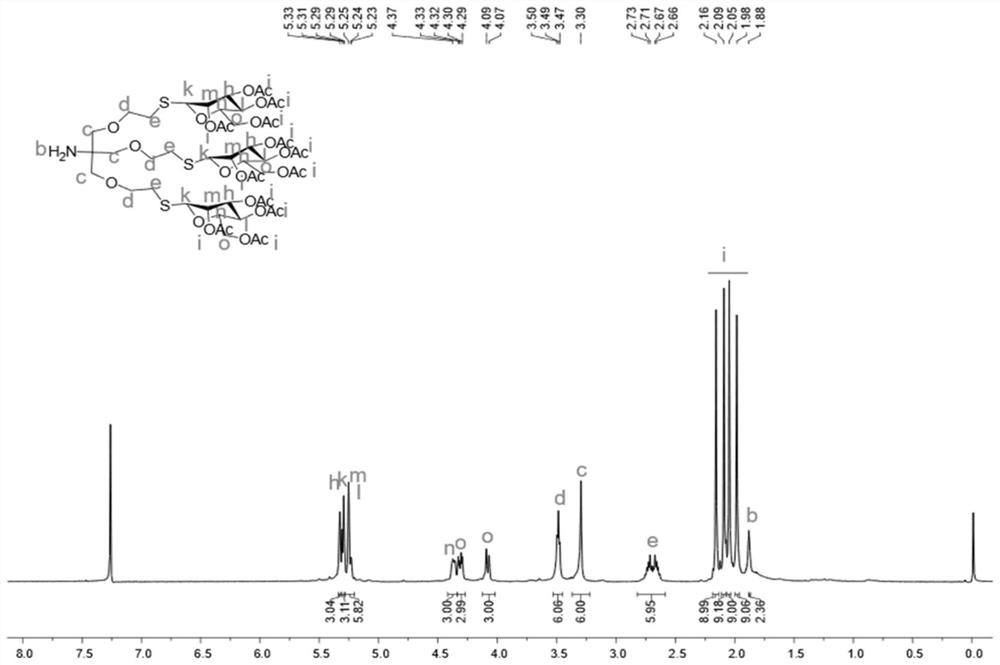
[0079] Compound C (1,3-bis(allyloxy)-2-(((prop-2-yn-1-yloxy)methyl)prop-2-yl)carbamate tert-butyl ester (1.3g ,3.9mmol) and azidomannose (α-Man-N 3 ) (1.6g, 4.3mmol) dissolved in 20mL t-BuOH / H 2 O (1:1 v / v) mixed solution, then added sodium ascorbate (0.8g, 3.9mmol) and copper sulfate pentahydrate (0.5g, 2.0mmol), stirred at room temperature for 5h. After TLC monitoring until the end of the reaction, the reaction solution was washed with dichloromethane (50mL×3) and saturated brine (50mL×3) respectively, and the organic phase collected after extraction was dried with anhydrous sodium sulfate, filtered, and depressurized Concentrated by distillation and purified by column chromatography, 2.6 g of a light yellow viscous product was obtained with a yield of 94%.
[0080] The H NMR spectrum and the C NMR spectrum of the obtained compound D can be found in Figure 5 and Figure 6 shown.
[0081] 1 H NMR (500MHz, CDCl 3 ):δ=7.71(s,1H),5.98–5....
Embodiment 3
[0091] Synthesis of Compound G
[0092] Compound F (tert-butyl (1-(allyloxy)-3-(prop-2-yn-1-yloxy)-2-((prop-2-yn-1-yloxy)methyl propyl-2-yl) carbamate (0.5g, 1.5mmol) and azidomannose (α-Man-N 3 ) (1.2g, 3.1mmol) dissolved in 20mL t-BuOH / H 2 O (1:1 v / v) mixed solution, then added sodium ascorbate (0.3g, 1.5mmol) and copper sulfate pentahydrate (0.2g, 0.8mmol), stirred at room temperature for 5h. After TLC monitoring until the end of the reaction, the reaction solution was washed with dichloromethane (50mL×3) and saturated brine (50mL×3) respectively, and the organic phase collected after extraction was dried with anhydrous sodium sulfate, filtered, and depressurized Concentrated by distillation and purified by column chromatography, 1.5 g of a light yellow viscous product was obtained with a yield of 94%.
[0093] The H NMR spectrum and the C NMR spectrum of the prepared compound G are shown in Figure 11 and Figure 12 shown.
[0094] 1 H NMR (500MHz, CDCl 3 ):δ=7.72(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com