Synthesis method of 1, 2, 4-oxadiazole-3, 5-diketone
A synthesis method and technology for oxadiazole, applied in 1 field, can solve the problems of high cost, complex production conditions and high equipment requirements, and achieve the effects of low production cost, fast reaction rate and low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
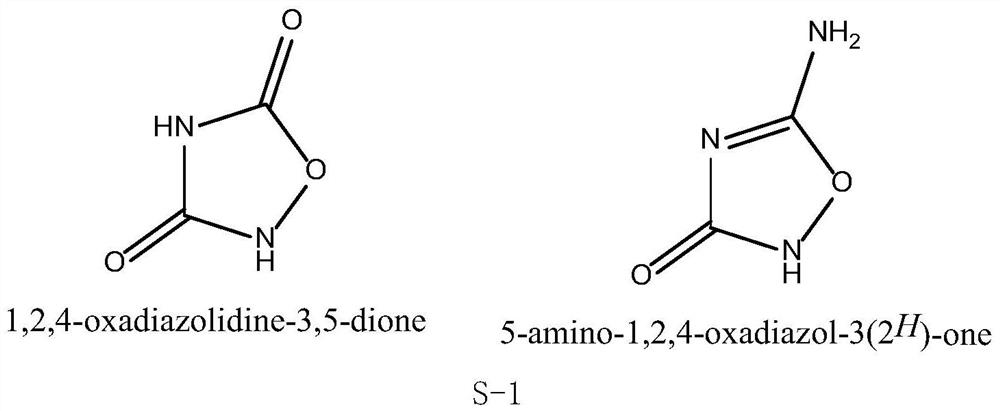
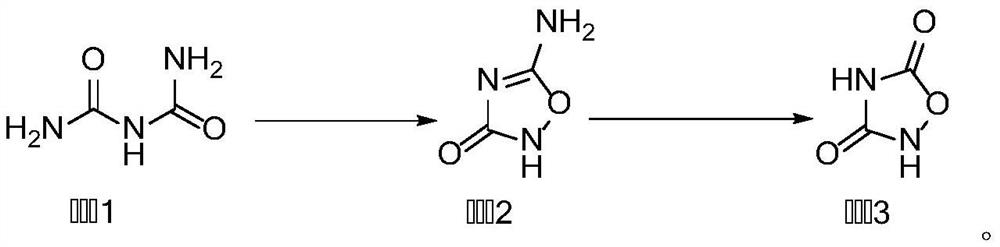
[0038] This embodiment provides a synthetic method of 1,2,4-oxadiazole-3,5-dione, comprising the following steps:
[0039] 1) Add compound 1 (10.3g, 0.1mol) and 170mL 1mol / L sodium hydroxide or potassium hydroxide aqueous solution into the flask under ice-water bath conditions. After the addition is complete, slowly add 30% hydrogen peroxide (15.9g) dropwise for 1h After the left and right additions are completed, react at 0°C for 45 minutes, adjust the pH to 4.5 with hydrochloric acid, a white precipitate appears, filter, and recrystallize the filter cake with pure water to obtain compound 2 with a yield of 87%;
[0040] 2) Add compound 2 (10.1g, 0.1mol), 90mL deionized water, and 40g acetic acid into the flask under ice-water bath conditions. After the addition is complete, slowly add 9.7g (0.14mol) of sodium nitrite. After the addition is complete, TLC monitors Reaction, the reaction temperature is 0-5°C, and the reaction time is 8h. After the reaction is completed, the rea...
Embodiment 2
[0042] This embodiment provides a synthetic method of 1,2,4-oxadiazole-3,5-dione, comprising the following steps:
[0043] 1) Add compound 1 (10.3 g, 0.1 mol) and 150 mL of 1 mol / L sodium hydroxide or potassium hydroxide aqueous solution into the flask under ice-water bath conditions. After the addition is complete, slowly add 30% hydrogen peroxide (13.6 g) dropwise for 1 h After the left and right additions are completed, react at 0°C for 45 minutes, adjust the pH to 4.5 with hydrochloric acid, a white precipitate appears, filter, and recrystallize the filter cake with pure water to obtain compound 2 with a yield of 83%;
[0044] 2) Add compound 2 (10.1g, 0.1mol), 80mL of deionized water, and 30g of acetic acid into the flask under ice-water bath conditions. After the addition is complete, slowly add 8.3g (0.12mol) of sodium nitrite. After the addition is complete, TLC monitors Reaction, the reaction temperature is 0-5°C, and the reaction time is 8h. After the reaction is com...
Embodiment 3
[0046] This embodiment provides a synthetic method of 1,2,4-oxadiazole-3,5-dione, comprising the following steps:
[0047]1) Add compound 1 (10.3g, 0.1mol) and 200mL 1mol / L sodium hydroxide or potassium hydroxide aqueous solution into the flask under ice-water bath conditions, after the addition is complete, slowly add 30% hydrogen peroxide (18.2g) dropwise for 1h After the left and right additions are completed, react at 0°C for 45 minutes, adjust the pH to 4.5 with hydrochloric acid, a white precipitate appears, filter, and recrystallize the filter cake with pure water to obtain compound 2 with a yield of 89%;
[0048] 2) Add compound 2 (10.1g, 0.1mol), 120mL of deionized water, and 50g of acetic acid into the flask under ice-water bath conditions. After the addition, slowly add 11, 1g (0.16mol) of sodium nitrite. Monitor the reaction, the reaction temperature is 0-5°C, and the reaction time is 8 hours. After the reaction is completed, the reaction solution is placed in the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com