Dihydrofuran quinolinone derivative and preparation method thereof
A technology of dihydrofuran quinolinone and its derivatives, applied in the direction of organic chemistry, which can solve the problems of metal residues and cumbersome steps, and achieve the effects of mild conditions, simple methods, and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: the preparation method of the dihydrofuran quinolinone derivative of the present embodiment, carry out according to the following steps:
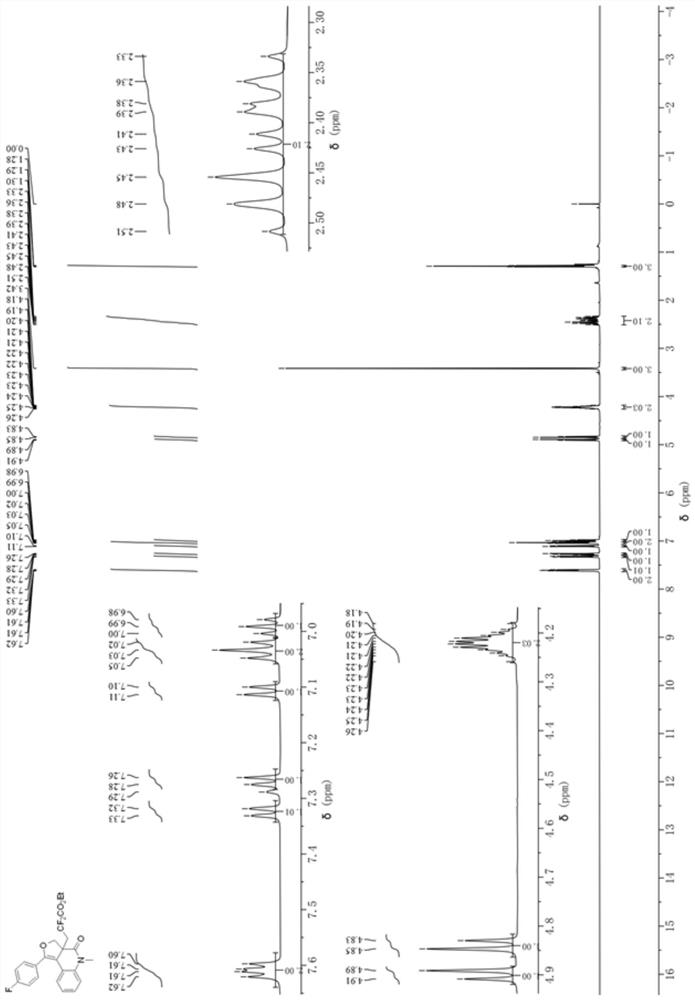
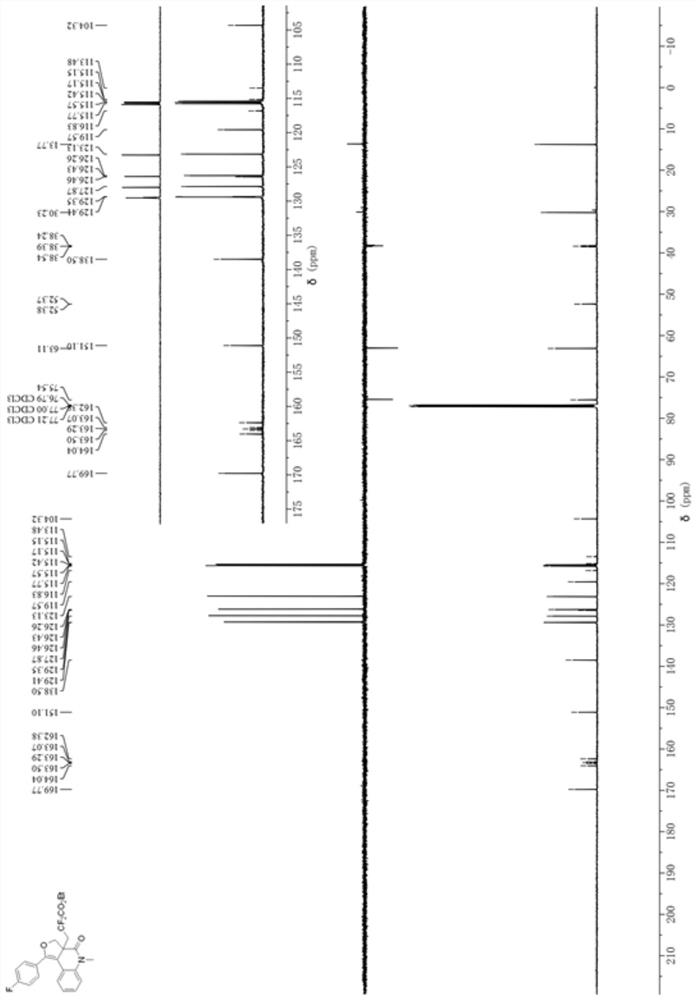
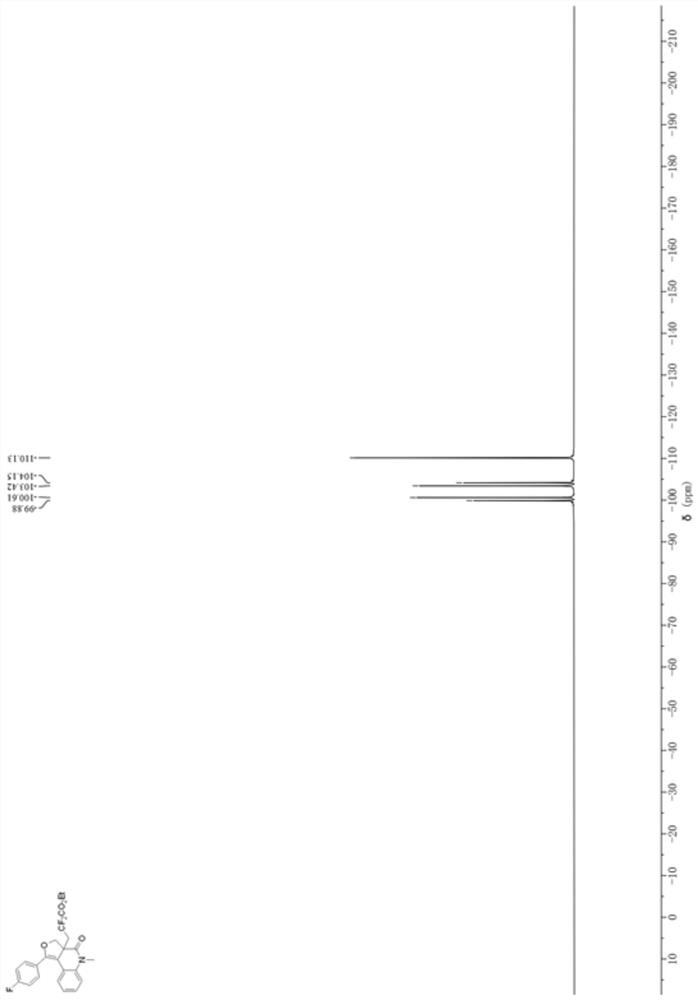
[0032] Add 0.0309 g (0.1 mmol) of the 1,7-enyne substrate (N-(2-(((4-fluorophenyl)ethynyl)phenyl)-2-(hydroxymethyl) to a 10 mL penicillin bottle -N-methacrylamide), 0.0304g (1.5mmol) of BrCF 2 CO 2 Et, 0.0032g mole percent concentration is the photocatalyst fac-Ir(ppy) of 5mol% 3 , 0.0652g (0.2mmol) of cesium carbonate; with a volume of 1mL of acetone as a reaction solvent, in N 2 Under the atmosphere, the reaction was stirred under 60W blue LED at room temperature for 2 h. After the reaction is completed, the reaction solvent is concentrated and spin-dried by a rotary evaporator, and then a mixed solution of petroleum ether: ethyl acetate with a volume ratio of 20:1 is used as an eluent to carry out purification and separation by silica gel column chromatography to obtain dihydrofuran Quinolinone derivatives. The ...
Embodiment 2
[0036] Embodiment 2: the preparation method of the dihydrofuran quinolinone derivative of the present embodiment, carry out according to the following steps:
[0037] Add 0.0305 g (0.1 mmol) of the 1,7-enyne substrate (2-(hydroxymethyl)-N-methyl-N-(2-(p-tolylethynyl)phenyl) to a 10 mL penicillin bottle Acrylamide), 0.0304g (1.5mmol) of BrCF 2 CO 2 Et, 0.0032g mole percent concentration is the photocatalyst fac-Ir(ppy) of 5mol% 3 , 0.0652g (0.2mmol) of cesium carbonate; with a volume of 1mL of acetone as a reaction solvent, in N 2 Under the atmosphere, the reaction was stirred under 60W blue LED at room temperature for 2 h. After the reaction is completed, the reaction solvent is concentrated and spin-dried by a rotary evaporator, and then a mixed solution of petroleum ether: ethyl acetate with a volume ratio of 20:1 is used as an eluent to carry out purification and separation by silica gel column chromatography to obtain dihydrofuran Quinolinone derivatives. The chemical...
Embodiment 3
[0041] Embodiment 3: the preparation method of the dihydrofuran quinolinone derivative of the present embodiment, carry out according to the following steps:
[0042] Add 0.0321 g (0.1 mmol) of 1,7-enyne substrate (2-(hydroxymethyl)-N-(2-(((4-methoxyphenyl)ethynyl)benzene base)-N-methacrylamide), 0.0304g (1.5mmol) of BrCF 2 CO 2 Et, 0.0032g (5mol%) photocatalyst fac-Ir (ppy) 3 , 0.0652g (0.2mmol) of cesium carbonate; with a volume of 1mL of acetone as a reaction solvent, in N 2 Under the atmosphere, the reaction was stirred under 60W blue LED at room temperature for 2 h. After the reaction is completed, the reaction solvent is concentrated and spin-dried by a rotary evaporator, and then a mixed solution of petroleum ether: ethyl acetate with a volume ratio of 20:1 is used as an eluent to carry out purification and separation by silica gel column chromatography to obtain dihydrofuran Quinolinone derivatives, its reaction formula is:
[0043]
[0044] The purity of the p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com