Application of Pityriacitrin alkaloid and derivative thereof in resisting plant viruses and pathogenic bacteria
A technology of anti-plant virus and anti-plant virus agent, applied in botany equipment and methods, chemicals for biological control, applications, etc., can solve the problem of low synthesis yield, high cost, low natural content of pityriacitrins alkaloids, etc. Problems, to achieve the effect of expanding the scope of application, good anti-plant virus and bacteria activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
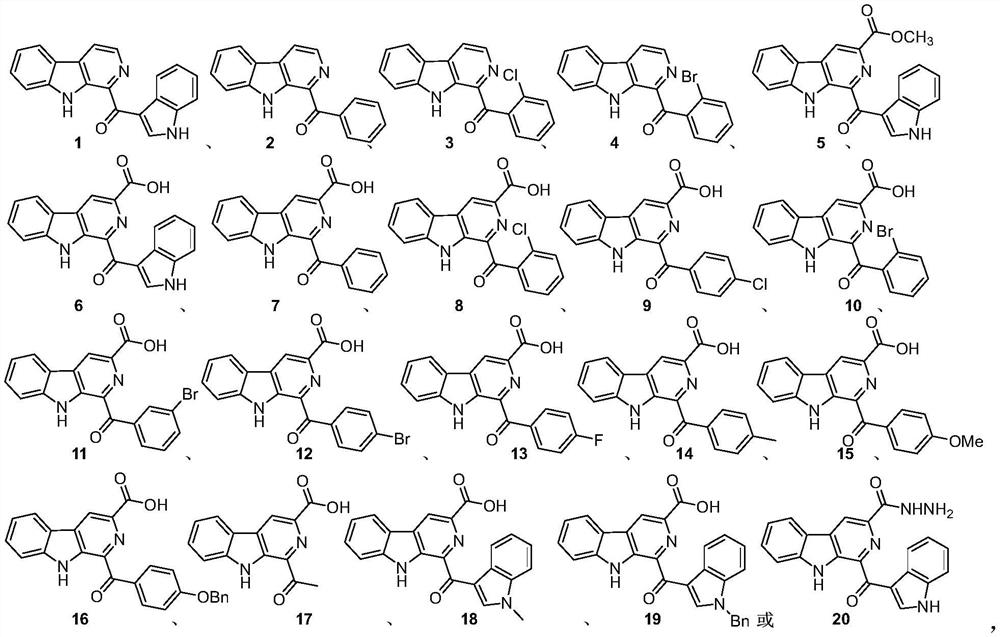
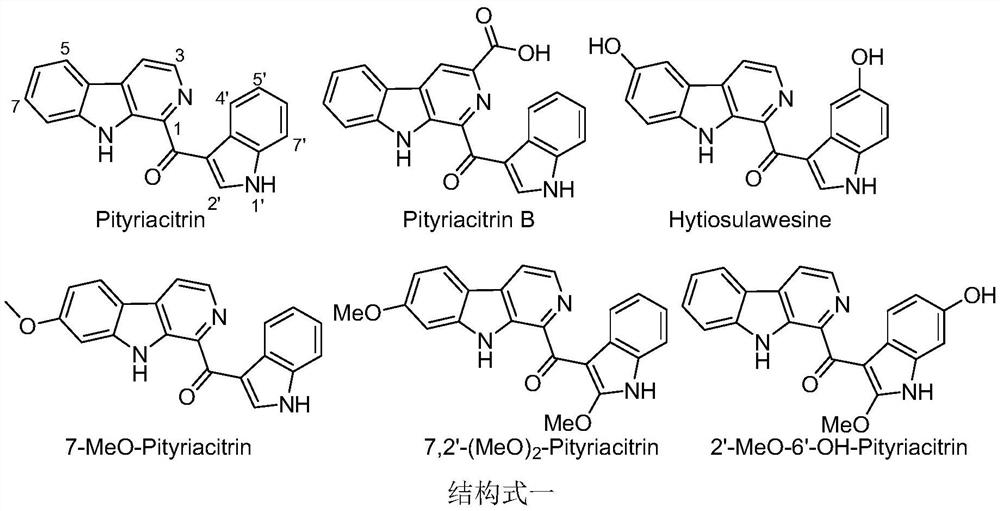
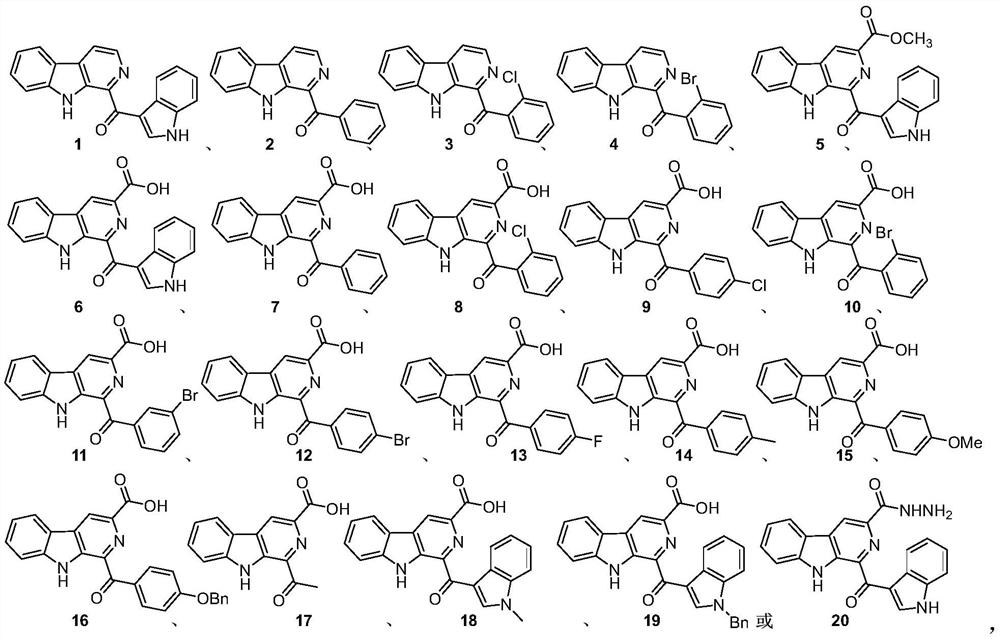
[0015] The pityriacitrin alkaloids and their derivatives represented by the following 1 to 20 chemical structural formulas of the present invention are obtained by referring to existing literature (Chem.Eur.J.2013,19,10132-10137; RSC Adv.,2014,4,26258- 26263.) prepared by the method disclosed,
[0016]
[0017] in,
[0018] The compound shown in chemical structure formula 1 is pityriacitrin alkaloid,
[0019] The pityriacitrin derivative shown in chemical structural formula 2 is phenyl (9H-pyrido [3,4-b] indol-1-yl) ketone,
[0020] The pityriacitrin derivative shown in chemical structure 3 is (2-chlorophenyl) (9H-pyrido [3,4-b] indol-1-yl) ketone,
[0021] The pityriacitrin derivative shown in chemical structure 4 is (2-bromophenyl) (9H-pyrido [3,4-b] indol-1-yl) ketone,
[0022] The pityriacitrin B derivative shown in chemical structural formula 5 is 1-(1H-indole-3-carbonyl)-9H-pyrido[3,4-b]indole-3-carboxylic acid methyl ester,
[0023] Chemical structural formula 6 ...
Embodiment 2
[0039] The individual compounds of pityriacitrin alkaloids and derivatives thereof shown in the above 1 to 20 chemical structural formulas are used as anti-plant virus agents:
[0040] The measurement of the anti-tobacco mosaic virus activity of the individual compounds of pityriacitrin alkaloids and derivatives thereof shown in the above 1 to 20 chemical structural formulas, the measurement procedure is as follows:
[0041] The first step, tobacco mosaic virus purification and concentration determination:
[0042] Tobacco mosaic virus purification and concentration determination were carried out in accordance with the Tobacco Mosaic Virus SOP specification compiled by the Bioassay Laboratory of the Institute of Elements, Nankai University. After the virus crude extract was centrifuged twice with polyethylene glycol, the measured concentration was 20 μg / mL, and refrigerated at 4 °C spare;
[0043] In the second step, preparation of individual compound medicament solutions of ...
Embodiment 3
[0060] The individual compounds of pityriacitrin alkaloids and derivatives thereof shown in the above 1 to 20 chemical structural formulas are used as phytopathogenic fungicides,
[0061] A. In vitro bactericidal test, bacterial growth rate determination method (plate method):
[0062] The antibacterial activity test of the individual compounds of pityriacitrin alkaloids and derivatives thereof shown in the above 1 to 20 chemical structural formulas, in vitro bactericidal test, the determination procedure is as follows:
[0063] Determination of bacterial growth rate is the plate method: respectively dissolve 3 mg of the individual compounds of pityriacitrin alkaloids and their derivatives shown in the above 1 to 20 chemical structural formulas in 0.03 mL of acetone, and then use 200 μg / mL Tween 80 Dilute the aqueous solution to a test concentration of 50mg / kg, then draw 1mL of the drug solution and inject it into the corresponding Petri dish, then add 9mL of the medium, shake...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com