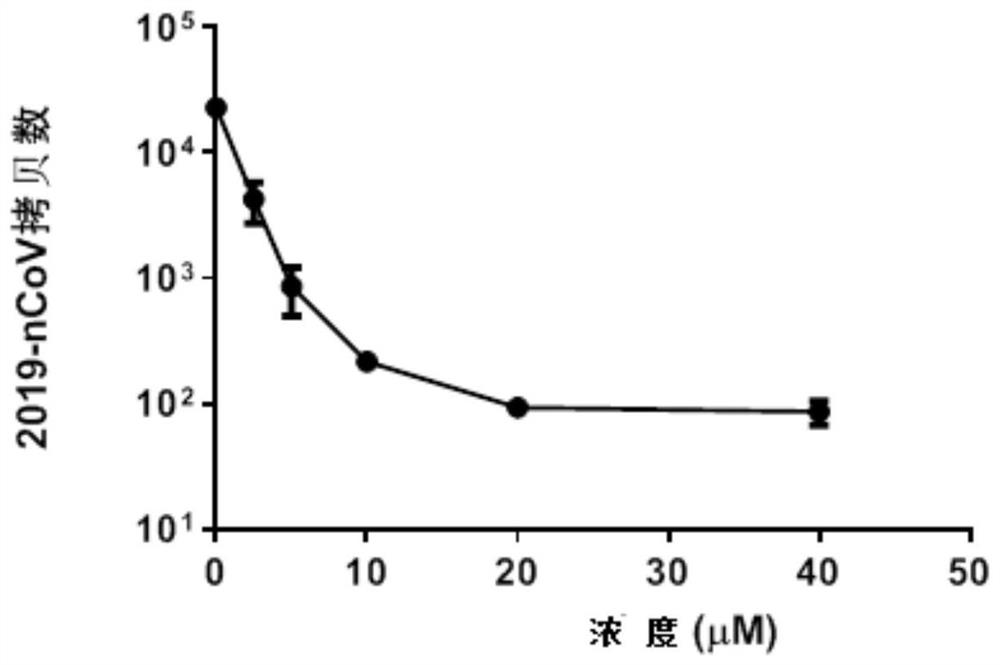
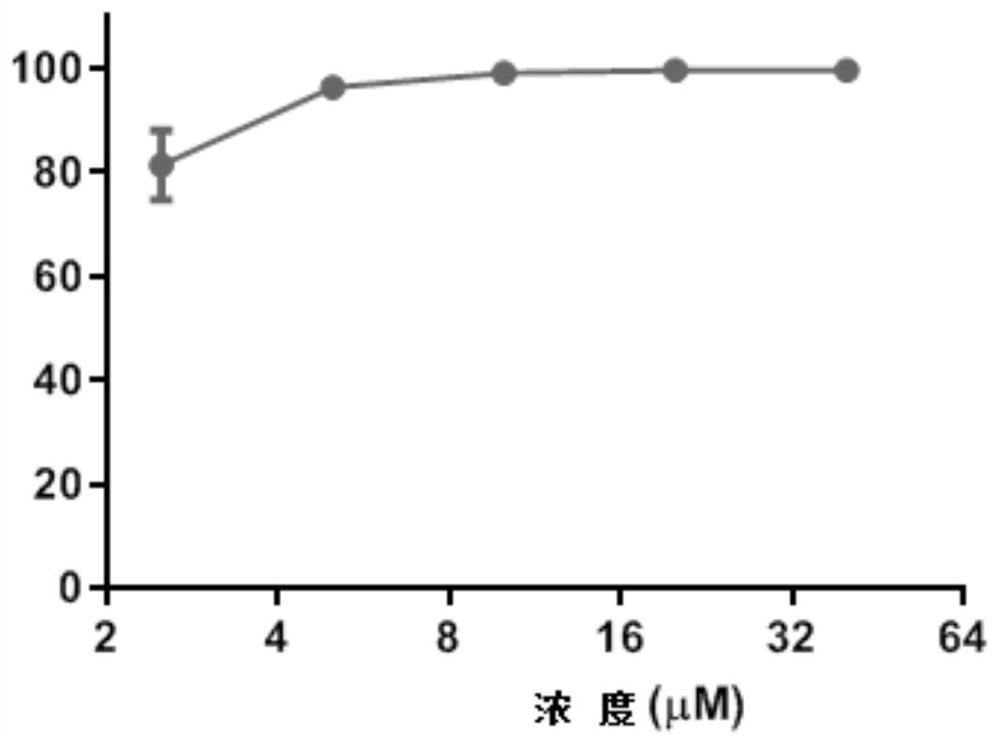
Application of 4-aminoquinoline compound
A technology of aminoquinoline and compounds, applied in the field of application of 4-aminoquinoline compounds, can solve the problems of high mortality rate in acute multiple organ failure, glucocorticoids cannot be regarded as ideal, and affect the rate of virus clearance, etc. Achieve the effect of preventing or treating acute immune inflammatory response, reducing local vascular permeability, and reducing pulmonary mucus exudation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] Embodiment 1 uses hydroxychloroquine to treat LPS-induced acute systemic inflammatory response (SIRS) in mice to improve survival rate
[0091] Using the method of intraperitoneal injection of lipopolysaccharide (Lipopolysaccharide, LPS), the systemic inflammation exudation mouse model was prepared, and the specific method steps were as follows:
[0092] 1. Grouping of mice: 8-12 weeks old female C57 mice (weight about 22-25g), after one week of adaptive feeding, were randomly divided into 5 groups according to the weight of the mice, namely the control group, the model group and the low-middle-high group Dosage Hydroxychloroquine group, 10 rats in each group.
[0093] 2. Preparation of SIRS mouse model by intraperitoneal injection of lipopolysaccharide: after dissolving lipopolysaccharide powder with phosphate buffered saline (PBS), intraperitoneal injection to mice at a ratio of 6 mg / kg (mg / kg) body weight to obtain an inflammatory mouse model , and the control group...
Embodiment 2
[0097] Example 2 Using hydroxychloroquine to treat multiple organ injuries in inflammatory mice can significantly prevent and reduce organ exudative inflammation
[0098] Oral gavage was used to administer medium and high doses of hydroxychloroquine to the mice with inflammation, and the model group and treatment group that were normal, dead, and at the end of the observation period were sacrificed and dissected, and their lungs, kidneys, spleen, For liver and intestinal tissues and organs, observe the specimens in general and then conduct HE staining pathological examination. The specific steps are as follows:
[0099] 1. Grouping of mice: 8-12 week-old female C57 mice (with a body weight of about 22-25g), after being adaptively fed for one week in an SPF animal room, were randomly divided into 5 groups according to the weight of the mice, respectively: Blank control (normal mice) group, inflammation mouse model group, hydroxychloroquine medium-dose, high-dose prophylactic dr...
Embodiment 3
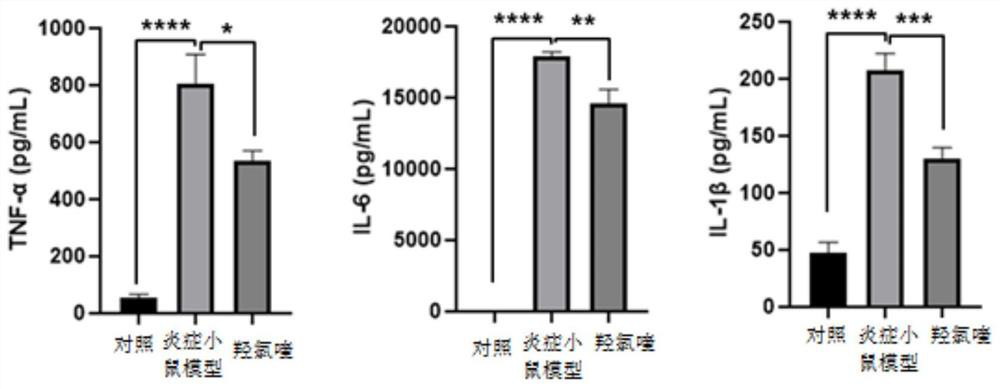
[0105] Example 3 Use of hydroxychloroquine to improve abnormal inflammatory response in inflammatory mice
[0106] 3.1 Administration of hydroxychloroquine was administered to inflammatory mice by intragastric administration, peripheral blood was taken for blood routine blood cell level (including lymphocytes, neutrophils, etc.) detection, and the peripheral blood of mice was determined by Elisa The level of relevant inflammatory factors, the specific steps are as follows:
[0107] 1. Grouping of mice: 8-12 week-old female C57 mice (with a body weight of about 22-25g) were fed adaptively for one week in an SPF animal room, and were randomly divided into 4 groups according to the weight of the mice, respectively: Blank control (normal mice) group, inflammatory mouse model group, inflammatory mouse plus hydroxychloroquine group (medium dose, high dose group), the number of each group is 10.
[0108] 2. Intraperitoneal injection of lipopolysaccharide (LPS): add hydroxychloroquin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com