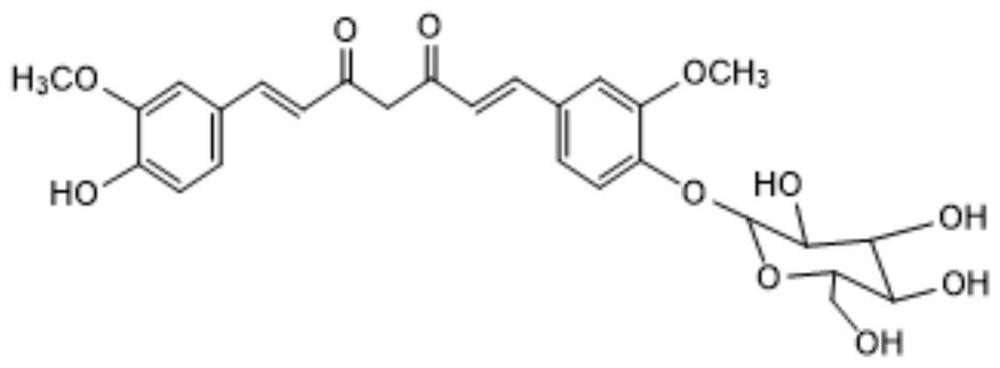
Method for catalytically synthesizing curcumin glycoside compounds by biological method
A technology of curcumin glycoside and biological method, which is applied in the field of bioengineering and can solve the problems of poor water solubility and low bioavailability of curcumin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
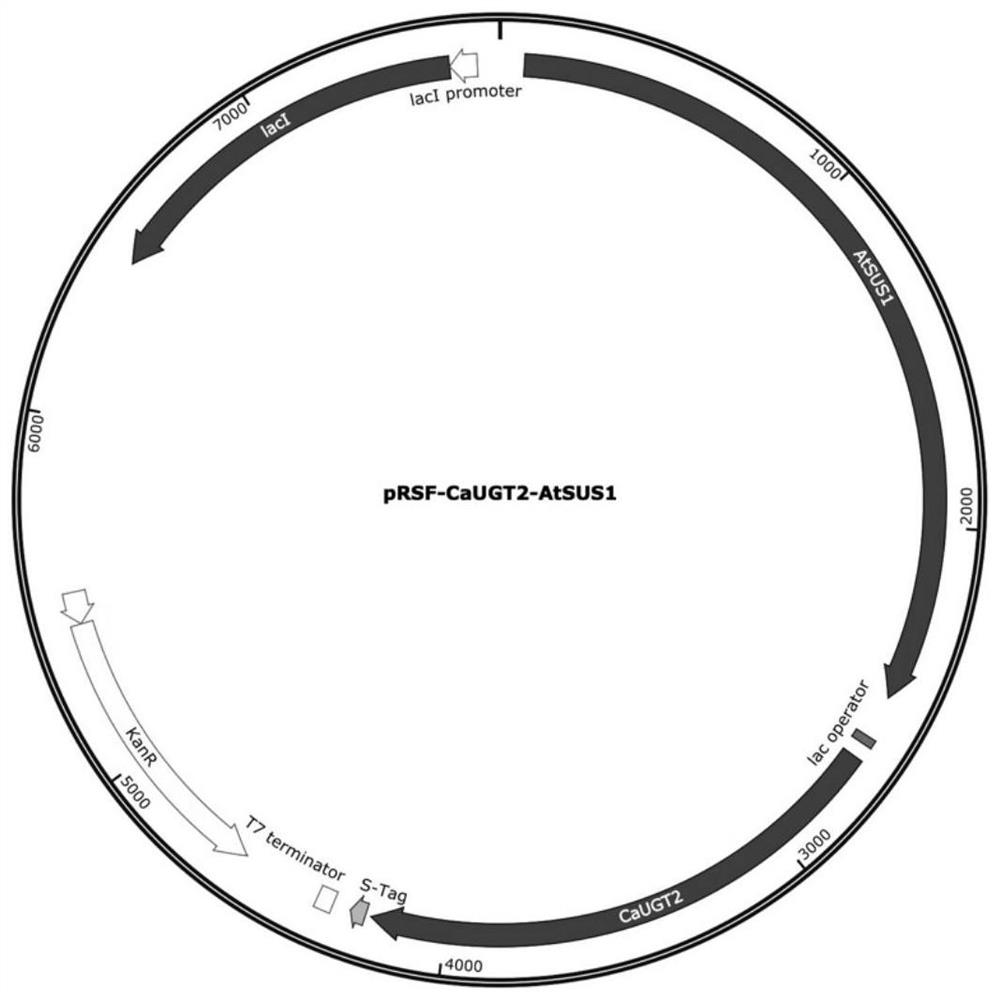
[0030] Example 1 Construction of recombinant plasmids
[0031] Select the double gene expression vector pRSFDuet-1, the recombinant plasmid is pRSF-CaUGT2-AtSUS1, in Nco I and EcoR I Insert the optimized sucrose synthase gene AtSUS1 , the sequence is shown in SEQ ID NO:1; in xho I and Nde I inserted the optimized glycosyltransferase gene CaUGT2 , the sequence is shown in SEQ ID NO: 3, and the recombinant plasmid pRSF-CaUGT2-AtSUS1 was obtained to form the co-expression recombinant plasmid pRSF-CaUGT2-AtSUS1. The recombinant plasmid was synthesized by Nanjing GenScript Company. sucrose synthase AtSUS1 Its amino acid sequence is shown in SEQ ID NO: 2, glycosyltransferase CaUGT2 Its amino acid sequence is shown in SEQ ID NO:4.
Embodiment 2
[0033] Example 2 Obtaining of Recombinant Escherichia coli Strains
[0034] The above-mentioned recombinant plasmid pRSF-CaUGT2-AtSUS1 was transformed into Escherichia coli BL21 (DE3) competent cells, and the transformants were spread on LB solid plates containing 50 µg / L kanamycin (NaCl 10 g / L, yeast powder 5 g / L peptone, 10 g / L peptone, 20 g / L agar), and cultured overnight in a 37°C incubator at a constant temperature to obtain a recombinant strain CaUGT2-AtSUS1 containing a dual-enzyme co-expression system.
[0035] The above recombinant plasmid pRSF-UGT76G1-StSUS1 was transformed into Escherichia coli BL21(DE3) competent cells, and the transformants were spread on LB solid plates containing 50 µg / L kanamycin (NaCl 10 g / L, yeast powder 5 g / L peptone, 10 g / L peptone, 20 g / L agar) and cultured overnight in a 37°C incubator at a constant temperature to obtain a recombinant strain UGT76G1-StSUS1 containing a dual-enzyme co-expression system.
Embodiment 3
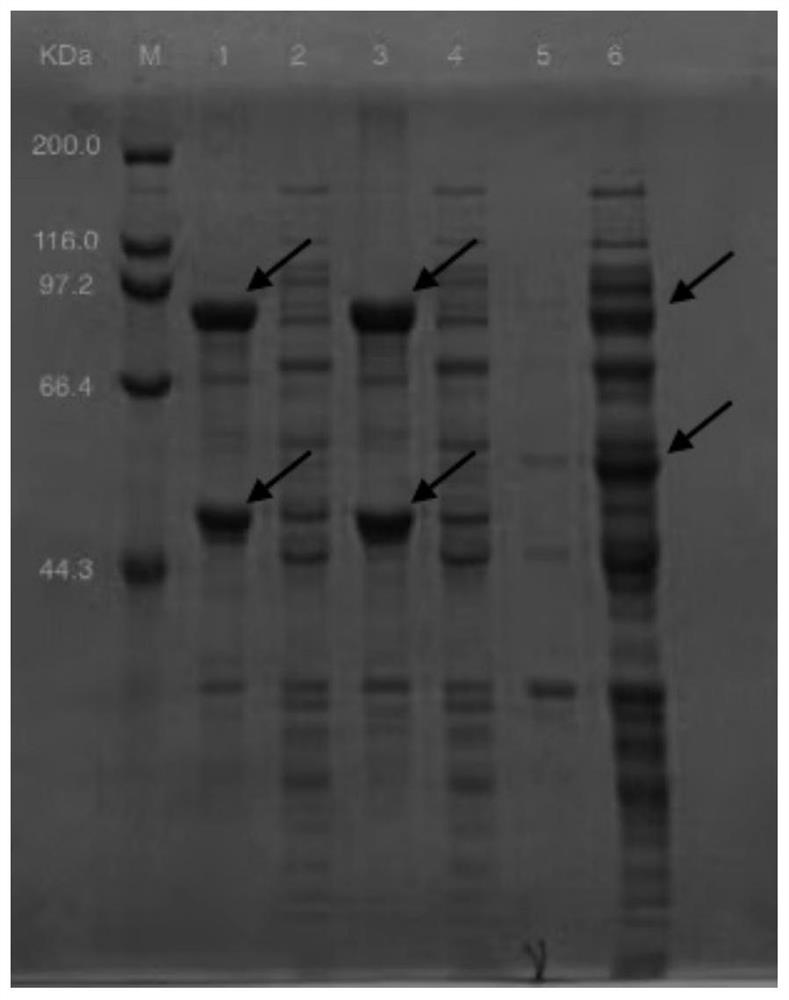
[0036] Example 3 Induced Expression of Recombinant Strains
[0037] The two recombinant strains constructed in Example 2 were respectively activated, transferred to LB medium, and placed in a shaker at 25-40°C, 200rpm, until the OD 600 When it reaches 0.6, add 0.1 mM inducer isopropyl-β-D-thiogalactoside to induce culture, centrifuge at low temperature (4°C, 7000 rpm, 6 min) and collect the bacteria, wash twice with potassium phosphate buffer. Then add an appropriate amount of potassium phosphate buffer, place it in an ice-water mixture, and use a sonicator to sonicate the bacteria, with the parameters set at Ф6, 300 W, and 30 min. Then centrifuge in a refrigerated centrifuge with the parameters set at 4°C, 8000rpm, for 30 min. The supernatant is taken as the crude enzyme solution, and stored in a 4°C refrigerator for later use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com