Arginine fluorescent probe and preparation method and application thereof
An arginine, optical probe technology, applied in the field of optical probes, which can solve the problems of in situ, real-time, dynamic, high-throughput and high spatiotemporal resolution detection of non-viable cells and subcellular organelles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
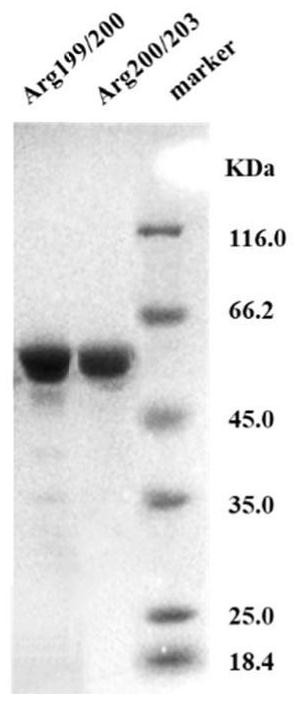
[0136] Example 1: Arginine-binding protein particles
[0137] The STM4351 gene in the Agrobacterium agrobacterium gene was amplified by PCR, and the PCR product was recovered after gel electrophoresis and digested with BamHI and EcoRI, and the pET28a vector was subjected to the same double digestion. After ligation with T4 DNA ligase, the ligation product was transformed into Trans5a, and the transformed Trans5a was spread on an LB plate (kanamycin 100ug / mL), and cultured at 37°C overnight. After plasmid extraction of the growing Trans5a transformants, PCR identification was performed. After the positive plasmid is correctly sequenced, the subsequent plasmid construction is carried out.
Embodiment 2
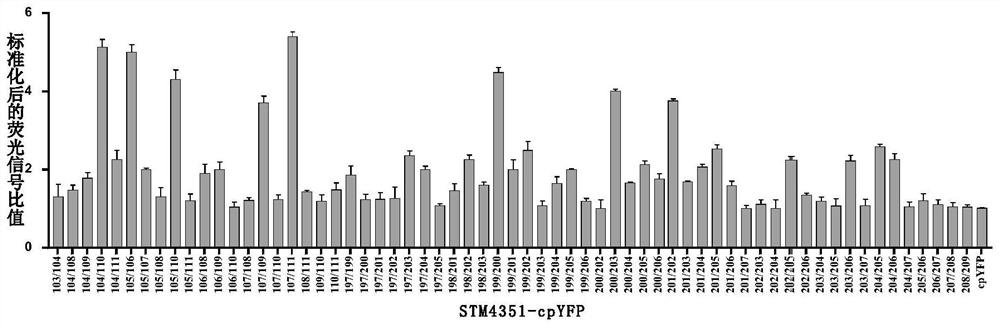
[0138] Example 2: Expression and detection of cpYFP optical probes at different insertion sites
[0139] In this example, based on pET28a-STM4351, the following sites were selected to insert cpYFP according to the crystal structure of arginine-binding protein, and the corresponding plasmids were obtained: 103 / 104, 103 / 105, 103 / 106, 103 / 107, 103 / 108, 103 / 109, 103 / 110, 103 / 111, 104 / 105, 104 / 106, 104 / 107, 104 / 108, 104 / 109, 104 / 110, 104 / 111, 105 / 106, 105 / 107, 105 / 108, 105 / 109, 105 / 110, 105 / 111, 106 / 107, 106 / 108, 106 / 109, 106 / 110, 106 / 111, 107 / 108, 107 / 109, 107 / 110, 107 / 111, 108 / 109, 108 / 110, 108 / 111, 109 / 110, 109 / 111, 110 / 111, 197 / 198, 197 / 199, 197 / 200, 197 / 201, 197 / 202, 197 / 203, 197 / 204, 197 / 205, 197 / 206, 197 / 207, 197 / 208, 197 / 209, 198 / 199, 198 / 200, 198 / 201, 198 / 202, 198 / 203, 198 / 204, 198 / 205, 198 / 206, 198 / 207, 198 / 208, 198 / 209, 199 / 200, 199 / 201, 199 / 202, 199 / 203, 199 / 204, 199 / 205, 199 / 206, 199 / 207, 199 / 208, 199 / 209, 200 / 201, 200 / 202, 200 / 203, 200 / 204, 200 / 205, 200 / 206, 200...
Embodiment 3
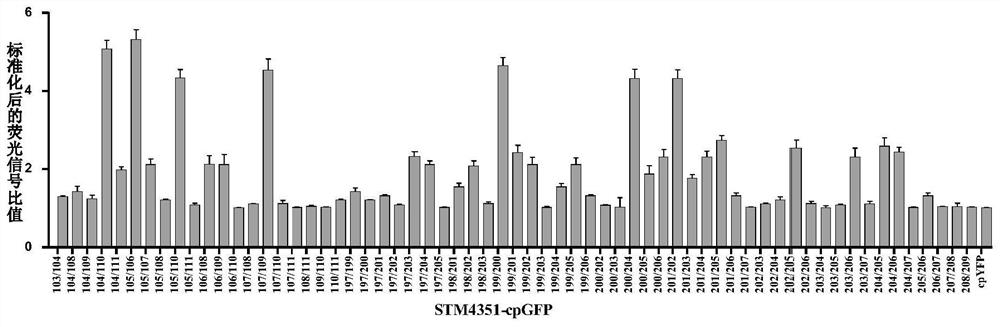
[0145] Example 3, Expression and detection of cpGFP optical probes at different insertion sites
[0146] According to the method in Example 1, cpYFP was replaced by green fluorescent protein cpGFP, which was fused into an arginine-binding protein to construct an arginine green fluorescent protein fluorescent probe, which was expressed and detected according to the method in Example 2. The result is as image 3 As shown, the results of fluorescence detection showed that 104 / 110, 105 / 106, 105 / 109, 105 / 110, 105 / 111, 106 / 108, 106 / 109, 107 / 109, 107 had more than 2 times response to arginine / 111, 197 / 203, 198 / 201, 199 / 200, 199 / 202, 199 / 203, 199 / 204, 200 / 202, 200 / 203, 200 / 204, 200 / 205, 200 / 206, 201 / 202 , 201 / 205, 204 / 205 sites.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com