Medicine composition, and preparation method and application thereof
A composition and drug technology, applied in the field of medicine, can solve problems such as complex mechanisms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0057] 1. Preparation and characterization of gene delivery system: Cationic polymer PLL-RT, targeting molecule HA and negatively charged gene material can be assembled into nanoparticles through electrostatic interaction, and the surface morphology of gene delivery system can be characterized by particle size analyzer and scanning electron microscope appearance.
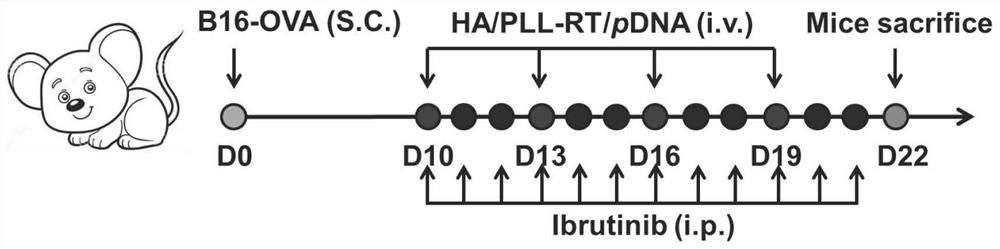
[0058] 2. Anti-tumor experiment: The co-administration of HA / PLL-RT / pSpam-1 / shPD-L1 nanoparticles and the small molecule inhibitor ibrutinib can exert the best anti-tumor effect.
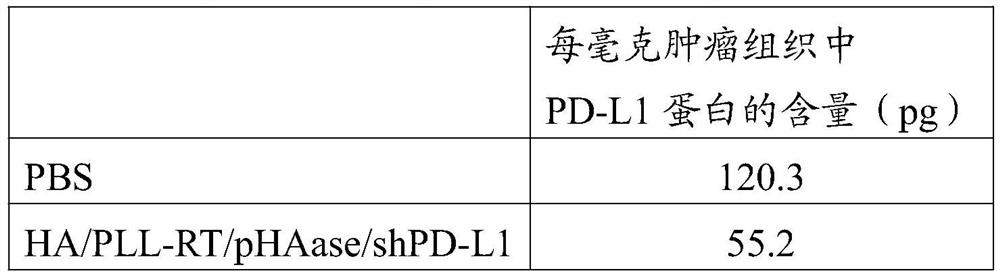
[0059] 3. Degradation of tumor cell immune checkpoint experiment: HA / PLL-RT / shPD-L1 nanoparticles can efficiently reduce the level of PD-L1 protein expressed by tumor cells in vivo. The level of PD-L1 protein was detected by ELISA.
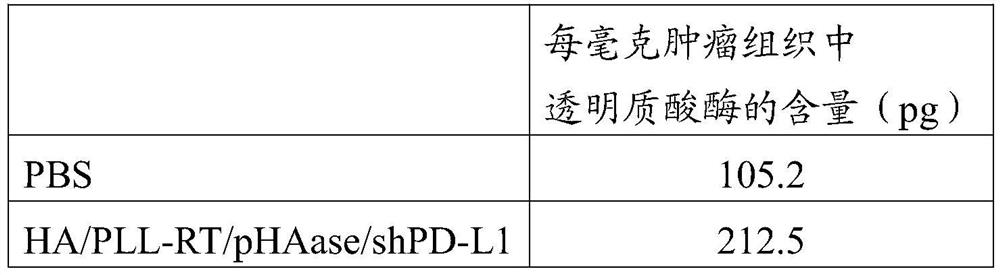
[0060] 4. Degradation of tumor extracellular matrix experiment: HA / PLL-RT / pSpam-1 nanoparticles can increase the expression of hyaluronidase in vivo, which is beneficial to the degradation of hyaluronic acid...
Embodiment 1 Embodiment 1
[0065] Embodiment 1 The preparation of embodiment 1PLL-RT gene carrier
[0066] Linear poly-α-lysine (molecular weight 15,000 Da) was dissolved in deionized water, and p-toluenesulfonyl and tert-butoxycarbonyl double-protected arginine was dissolved in DMF. Then, add EDC·HCl and HOBT, activate the reaction at room temperature for 1 hour, then slowly add the aqueous solution of PLL, and react at room temperature for 72 hours. After dialysis and freeze-drying, the product was reacted under the condition of trifluoroacetic acid for 4 hours, added with anhydrous ether to settle, vacuum-dried, dialyzed, and freeze-dried to obtain the white solid product PLL-RT.
[0067] The molar ratio of poly-α-lysine grafted to tosyl-protected arginine (Arg(Tos)) was 1:90.
Embodiment 2
[0068] Example 2 Preparation of HA / PLL-RT / pSpam-1 / shPD-L1 gene delivery system
[0069] The targeting molecule HA, cationic carrier PLL-RT, pSpam-1 and shPD-L1 were dissolved in ultrapure water to form aqueous solutions with concentrations of 0.1mg / mL, 1mg / mL, 0.2mg / mL and 0.2mg / mL, respectively. mL. Then PLL-RT, pSpam-1 and shPD-L1 were compounded in equal volumes, and the compounding ratio was 5:1:1. After vortexing for 30s, incubate at room temperature for 20 minutes, then add an equal volume of HA solution, vortex for 30s, incubate for another 20 minutes at room temperature, and finally obtain HA / PLL-RT / pSpam-1 / shPD- L1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com