A low-viscosity high-permeability polyaspartate
An aspartic ester, high penetration technology, applied in polyurea/polyurethane coatings, coatings, etc., can solve the problems of reducing, reducing the molecular chain strength, reducing the permeability of polyaspartic acid ester, and achieving low viscosity , increase cement strength, high flexibility effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
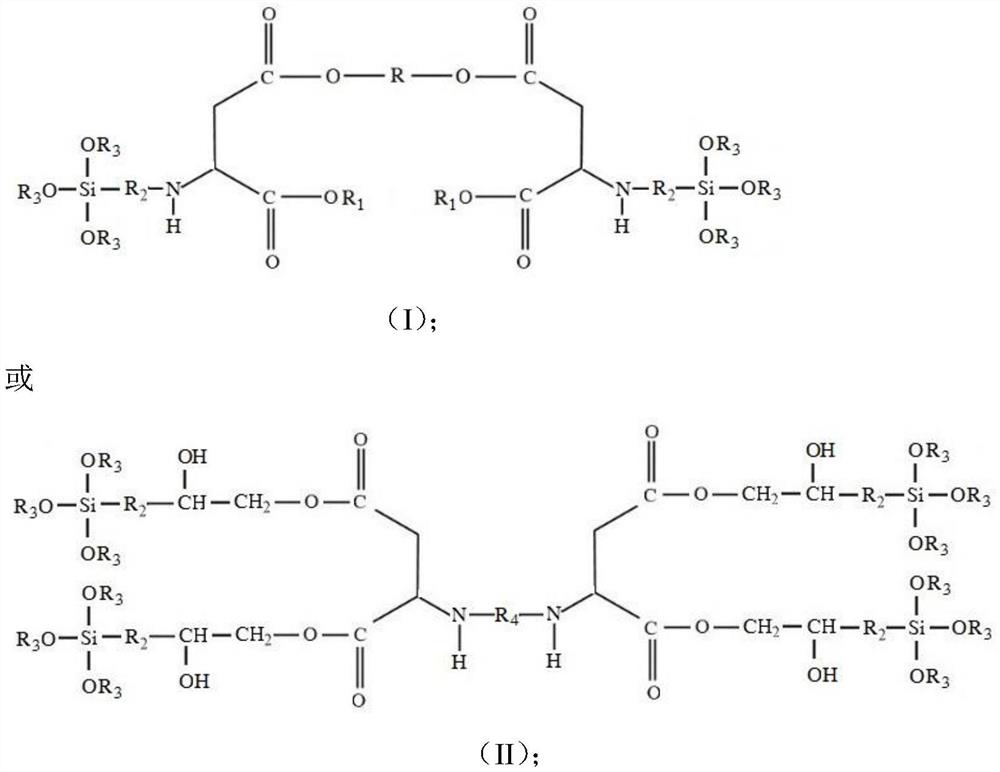
[0042] A low-viscosity high-permeability polyaspartic acid ester, the structural formula is:
[0043]
[0044] Among them, R and R 1 is ethyl; R 2 is n-propyl, R 3 For ethyl.
[0045] Its preparation method is:
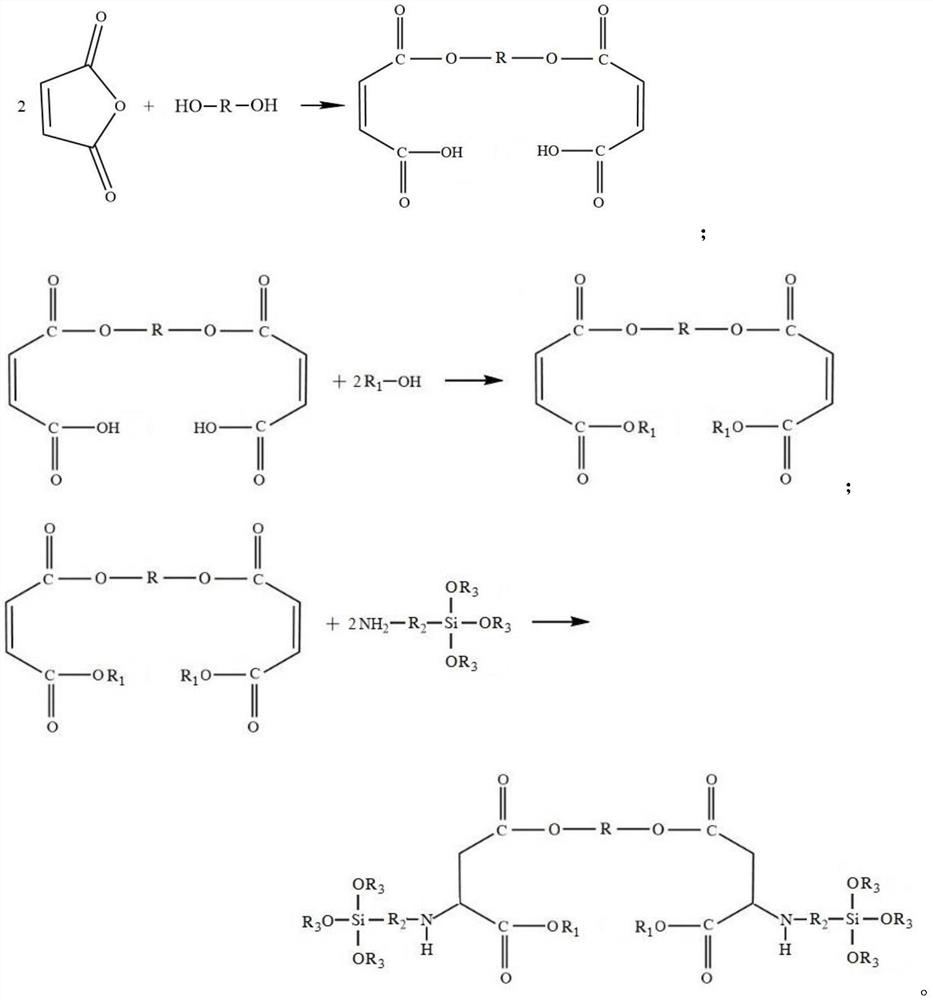
[0046] (1) Add maleic anhydride and ethylene glycol with a molar ratio of 2.0:1 into a flask with a water separator, and add 0.15% of the total mass of reactants as a polymerization inhibitor p-hydroxyanisole (MEHQ), at 150°C React for 5 hours under the protection of nitrogen, until the acid value does not change for 1 hour;
[0047] (2) adding ethanol that is 1:1.0 to the esterification product of step (1) with the maleic anhydride molar ratio, and finishing the reaction after reacting to the acid value<5mg KOH / g;
[0048] (3) Add gamma-aminopropyltriethoxysilane with a molar ratio of 0.98:1 to maleic anhydride to the product obtained in step (2), and catalyst sodium methylate with 0.03% of the total mass of reactants, 60°C under nitrogen React for 12 hours ...
Embodiment 2
[0050] A low-viscosity high-permeability polyaspartic acid ester, the structural formula is:
[0051]
[0052] Among them, R is a diethylene glycol group, R 1 is ethyl, R 2 is n-propyl, R 3 For ethyl.
[0053] Its preparation method is:
[0054] (1) Add maleic anhydride and diethylene glycol with a molar ratio of 1.8:1 into a flask with a water separator, and add a polymerization inhibitor MEHQ of 0.1% of the total mass of reactants, and react under nitrogen protection at 145°C 6h, until the acid value 1h no change;
[0055] (2) adding ethanol that is 1:0.9 to the esterification product of step (1) with the maleic anhydride molar ratio, and reacting to the acid value<5mg KOH / g to finish the reaction;
[0056] (3) Add gamma-aminopropyltriethoxysilane with a molar ratio of maleic anhydride of 0.95:1 to the product obtained in step (2), and catalyst sodium methoxide with 0.02% of the total mass of reactants, 55°C under nitrogen React for 16 hours under protection to obtain...
Embodiment 3
[0058] A low-viscosity high-permeability polyaspartic acid ester, the structural formula is:
[0059]
[0060] Among them, R 2 for -CH 2 -O-CH 2 -CH 2 -CH 2 -, R 3 is methyl, R 4 For ethyl.
[0061] Its preparation method is:
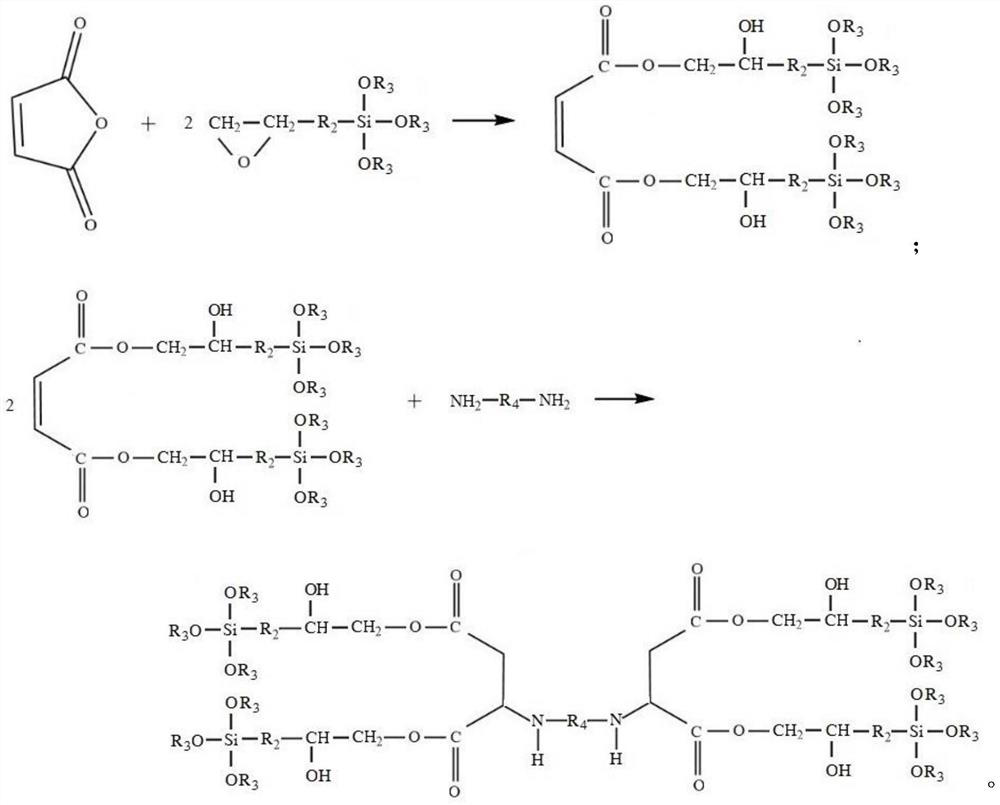
[0062] A) Add maleic anhydride and γ-glycidyl etheroxypropyltrimethoxysilane with a molar ratio of 1:2.2 into the flask, and add 0.15% of the total mass of the reactants to the polymerization inhibitor MEHQ and the total mass of the reactants to 0.08 % of the catalyst triphenylphosphine, under the protection of nitrogen at 100 ℃, the acid value is less than 5mg KOH / g, and the maleate is obtained;
[0063] B) Add ethylenediamine to the obtained maleic acid ester, the molar ratio of maleic acid ester to ethylenediamine is 2.2:1, and add 0.03% catalyst sodium methoxide of the total mass of reactants, under nitrogen protection at 60°C After 10 hours of reaction, the polyaspartic acid ester was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com