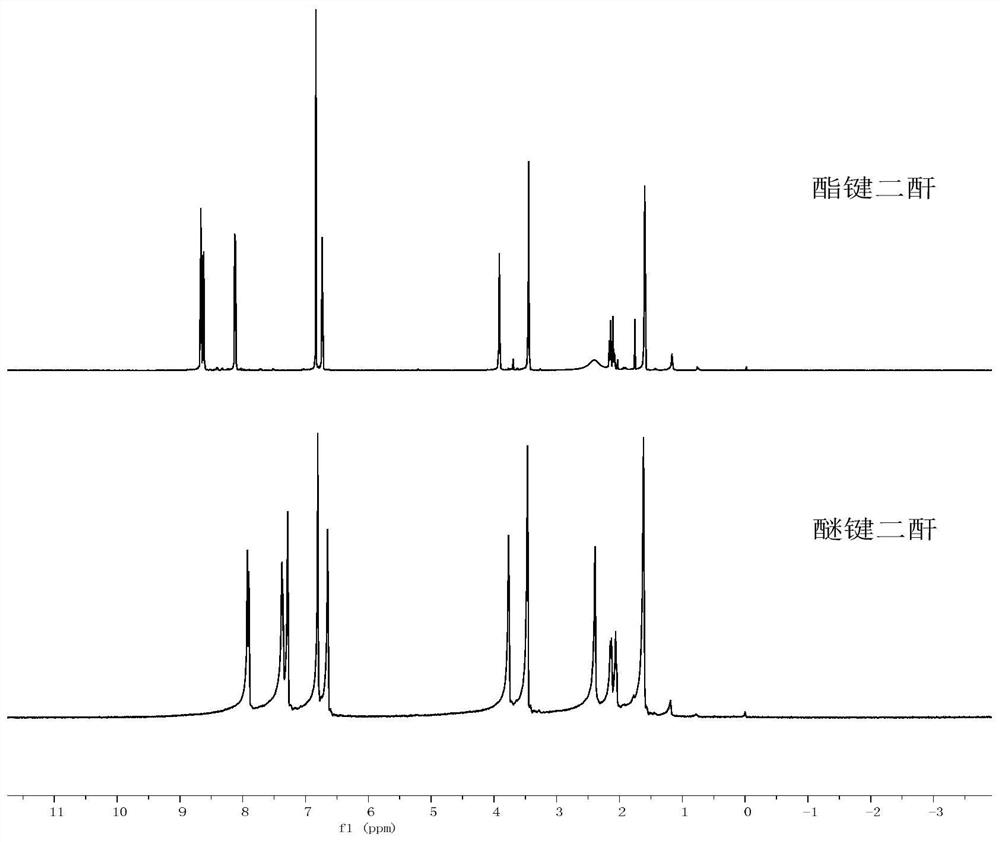
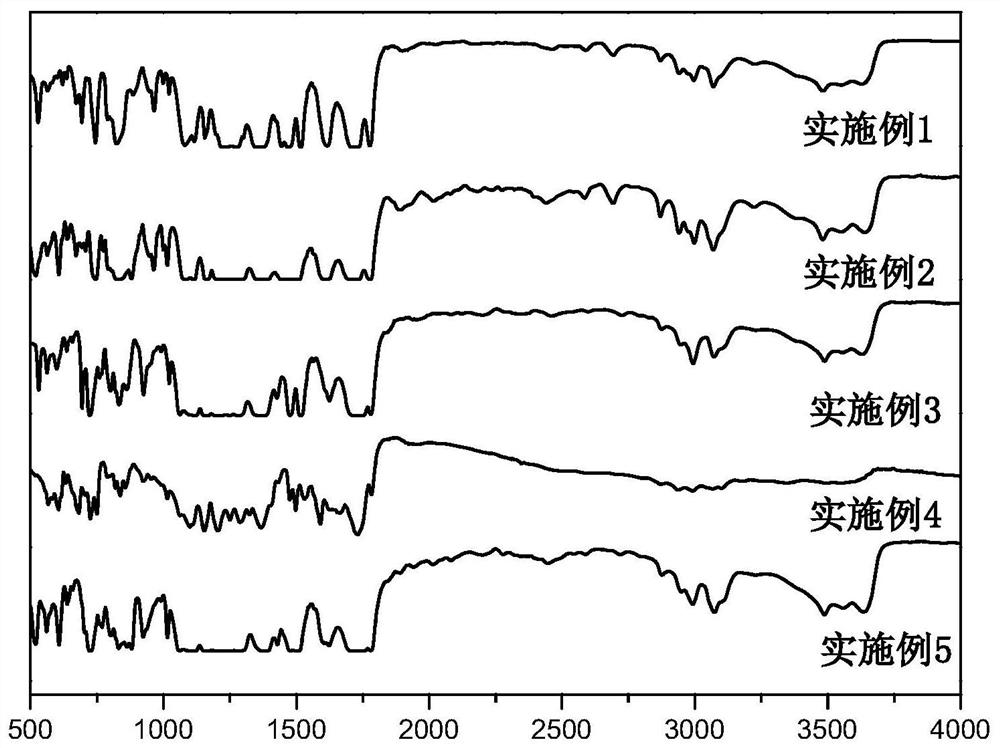
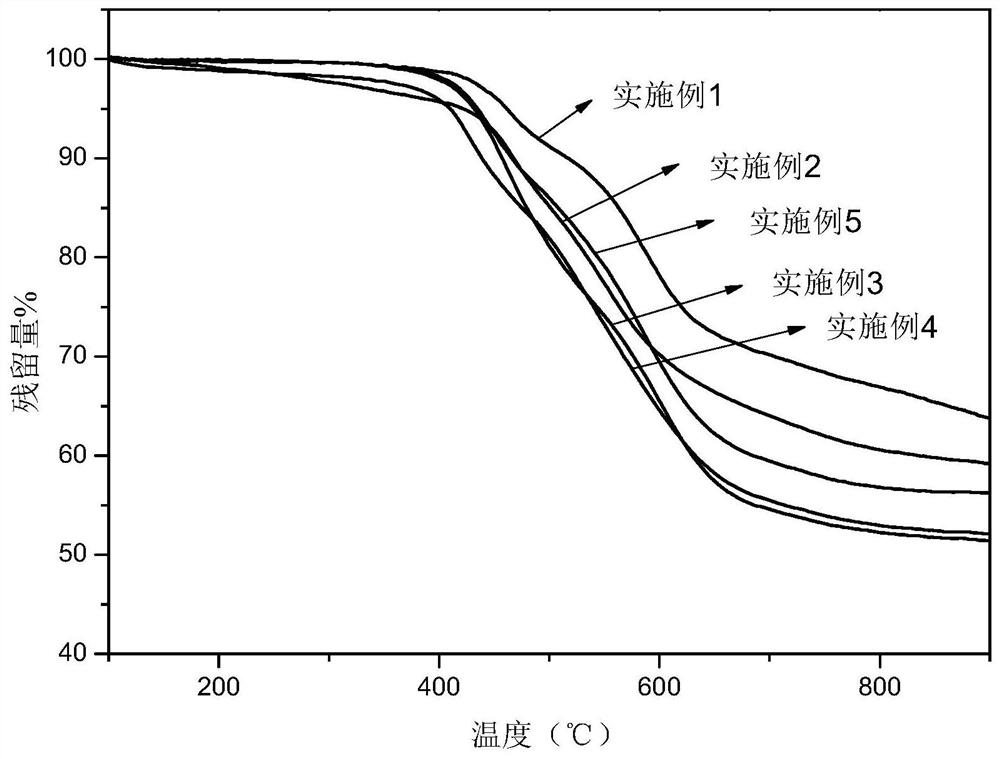
Polyimide with main chain containing benzo norbornene structure and preparation method thereof
A technology of benzonorbornene and polyimide, which is applied in the field of polyimide materials and preparation, can solve the problems of high glass transition temperature or melting temperature, processing difficulties, etc., and achieves reduction of close packing and excellent mechanical properties. , the effect of improving heat resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] (1) Preparation of diketone compound.
[0051] Under the conditions of nitrogen, ice bath and mechanical stirring, take benzoquinone compound 21.62g (200mmol) and 250mL acetone in a 500mL three-necked flask, dissolve 14.85mL (220mmol) cyclopentadiene compound in 60mL acetone in a constant pressure dropping funnel , under vigorous mechanical stirring, the cyclopentadiene solution was added dropwise to the 1,4-benzoquinone solution, kept for 1.5 hours, slowly raised to room temperature (25°C), and reacted at room temperature for 6 hours to obtain a brown solution. The acetone was removed by a rotary evaporator, and the remaining tan solid was recrystallized from n-hexane to precipitate 30.92 g of a yellow diketone compound.
[0052] (2) Preparation of a diphenol compound containing a benzonorbornene structure.
[0053] Under the condition of room temperature and magnetic stirring, get 10g (57mmol) diketone compound, 20g basic aluminum oxide and 200mL ethyl acetate in 250...
Embodiment 2
[0061] 1) Preparation of diketone compounds.
[0062] Under the conditions of nitrogen, ice bath and mechanical stirring, take 21.62g (200mmol) of benzoquinone compound and 350mL methanol in a 500mL three-necked flask, dissolve 14.2mL (210mmol) cyclopentadiene compound in 60mL methanol in a constant pressure dropping funnel , under vigorous mechanical stirring, the cyclopentadiene solution was added dropwise to the 1,4-benzoquinone solution, kept for 1.5 hours, slowly raised to room temperature (25°C), and reacted at room temperature for 6 hours to obtain a brown-yellow solution. Methanol was removed by a rotary evaporator, and the remaining tan solid was recrystallized from n-hexane to precipitate 30.12 g of a yellow diketone compound.
[0063] (2) Preparation of a diphenol compound containing a benzonorbornene structure.
[0064] Under the condition of room temperature and magnetic stirring, get 10g (57mmol) diketone compound, 15g basic aluminum oxide and 230mL ethyl acetat...
Embodiment 3
[0072] (1) Preparation of diketone compound.
[0073] Under the conditions of nitrogen, ice bath and mechanical stirring, take 21.62g (200mmol) of benzoquinone compound and 300mL methanol in a 500mL three-necked flask, dissolve 13.5mL (200mmol) cyclopentadiene compound in 50mL methanol in a constant pressure dropping funnel , under vigorous mechanical stirring, the cyclopentadiene solution was added dropwise to the 1,4-benzoquinone solution, kept for 1.5 hours, slowly raised to room temperature (25°C), and reacted at room temperature for 6 hours to obtain a brown solution. Methanol was removed by a rotary evaporator, and the remaining tan solid was recrystallized from n-hexane to precipitate 32.56 g of a yellow diketone compound.
[0074] (2) Preparation of a diphenol compound containing a benzonorbornene structure.
[0075] Under the condition of room temperature and magnetic stirring, get 10g (57mmol) diketone compound, 20g basic aluminum oxide and 200mL ethyl acetate in 25...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com