Liquid crystal compound, liquid crystal composition, and liquid crystal display device
A liquid crystal compound and liquid crystal composition technology, applied in liquid crystal materials, chemical instruments and methods, instruments, etc., can solve the problem of weak anchoring liquid crystal molecules, affecting the display effect of liquid crystal display devices, reducing the yield rate of liquid crystal display devices, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0120] In order to illustrate the present invention more clearly, the present invention will be further described below in conjunction with preferred embodiments. Those skilled in the art should understand that the content specifically described below is illustrative rather than restrictive, and should not limit the protection scope of the present invention.
[0121] In the present invention, the preparation method is a conventional method unless otherwise specified, and the raw materials used can be obtained from open commercial channels unless otherwise specified. The percentages refer to mass percentages, and the temperature is Celsius (°C). The meaning and test conditions are as follows:
[0122] Cp means liquid crystal clearing point (℃), DSC quantitative method test;
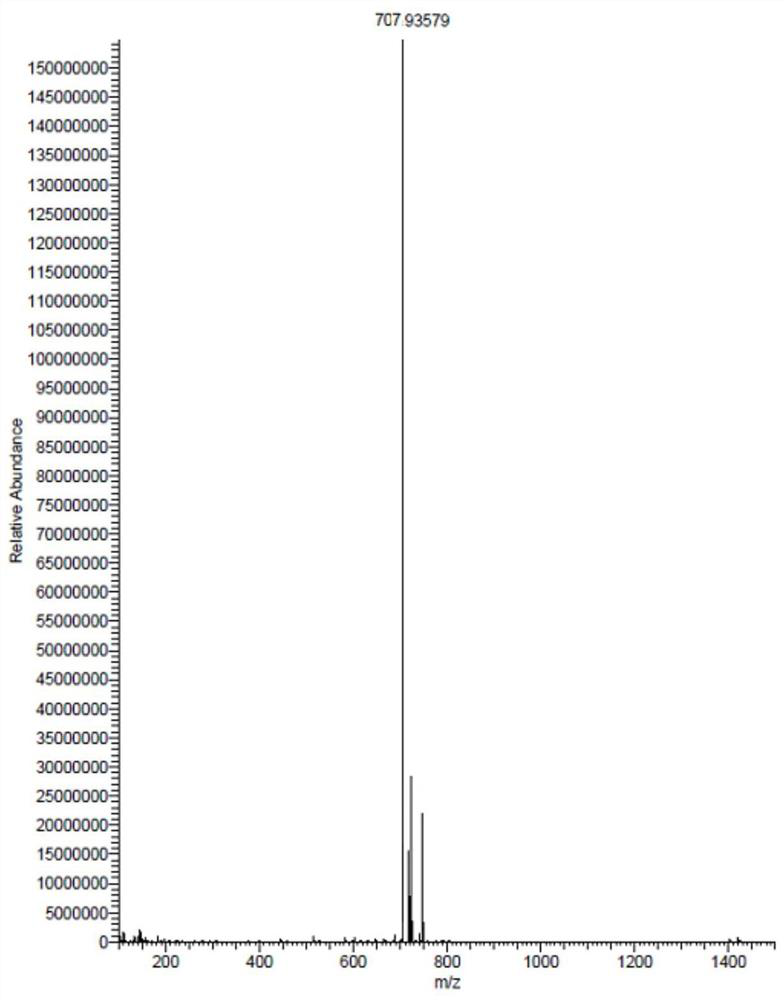
[0123] Δn means optical anisotropy, Δn=n e -n o , where n o is the refractive index of ordinary light, n e is the refractive index of extraordinary light, the test condition is 25±2°C, 589nm, Abbe ref...
Synthetic example 1
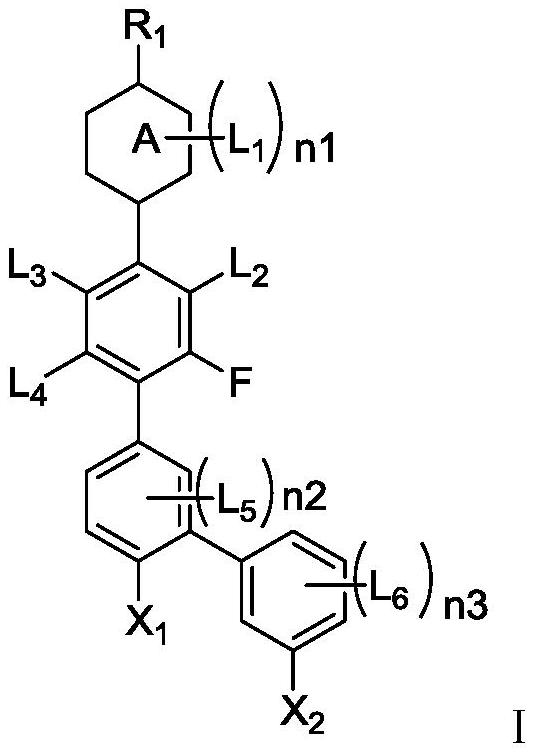
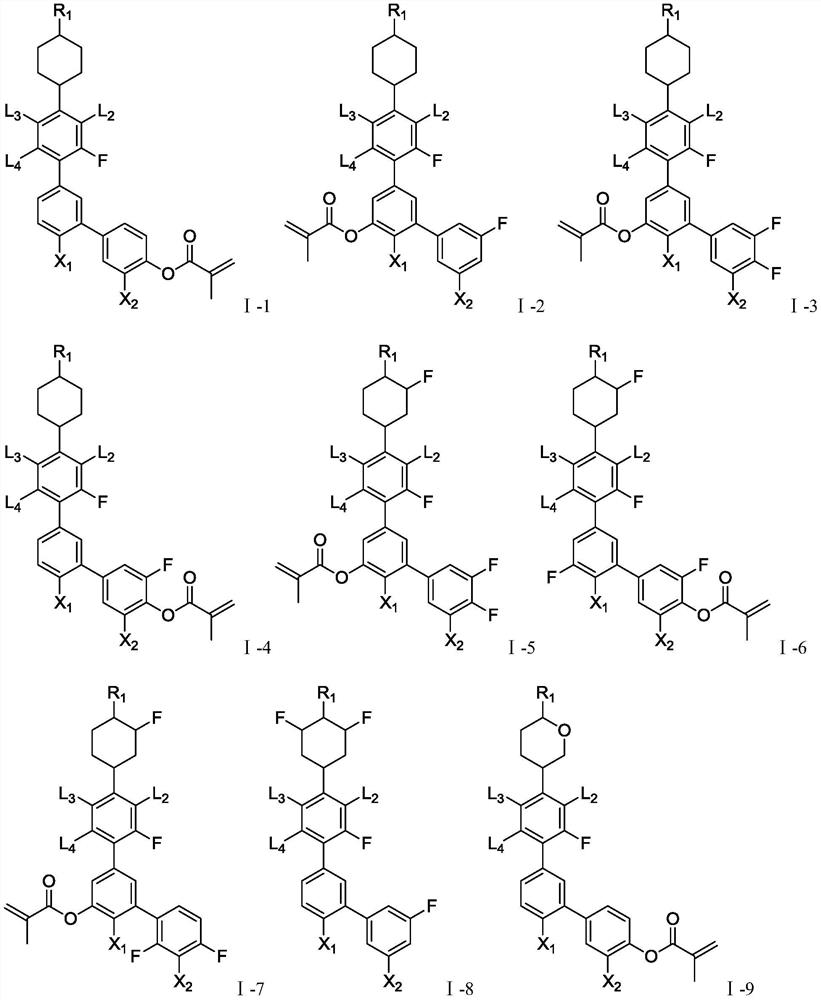
[0166] The structural formula of the compound is shown in the following formula I-1-1,
[0167]
[0168] Its preparation route is as follows:
[0169]
[0170] The specific operation process of preparation:
[0171] Intermediate 1
[0172] Under nitrogen protection, put 0.3mol of 2-benzyloxy-5-bromophenol, 0.39mol of 5-tert-butyldimethylsilyloxypentane bromide, and 0.39mol of anhydrous potassium carbonate into a 2L three-necked flask. 0.8LDMF, heated at 120°C for 3 hours. The reaction was monitored by a TLC plate. After the reaction was completed, the temperature was lowered, water was added with ethyl acetate, the liquid was separated, extracted and washed with water, the solvent was spin-dried, and separated by column chromatography to obtain compound 1 as a yellow solid. HPLC: 93%, yield Y=85%.
[0173] Intermediate 2
[0174] Under nitrogen protection, put 0.3mol of 4-pentylcyclohexyl 2,3-difluorophenylboronic acid, 0.3mol of o-bromo-p-iodophenol, 0.9L of toluen...
Synthetic example 2
[0188] The structural formula of the compound is shown in the following formula I-1-7,
[0189]
[0190] Its preparation route is as follows:
[0191]
[0192] The specific operation process of preparation:
[0193] Intermediate 1
[0194] The synthesis method refers to the synthesis of intermediate 3 in Example 1.
[0195] Intermediate 2
[0196] The synthesis method refers to the synthesis of intermediate 3 in Example 1.
[0197] Intermediate 3
[0198] The synthesis method refers to the synthesis of intermediate 3 in Example 1.
[0199] Intermediate 4
[0200] The synthesis method refers to the synthesis of intermediate 4 in Example 1.
[0201] Intermediate 5
[0202] The synthesis method refers to the synthesis of intermediate 5 in Example 1.
[0203] Intermediate 6
[0204] The synthesis method refers to the synthesis of intermediate 6 in Example 1.
[0205] Intermediate 7
[0206] The synthesis method refers to the synthesis of intermediate 7 in Example...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com