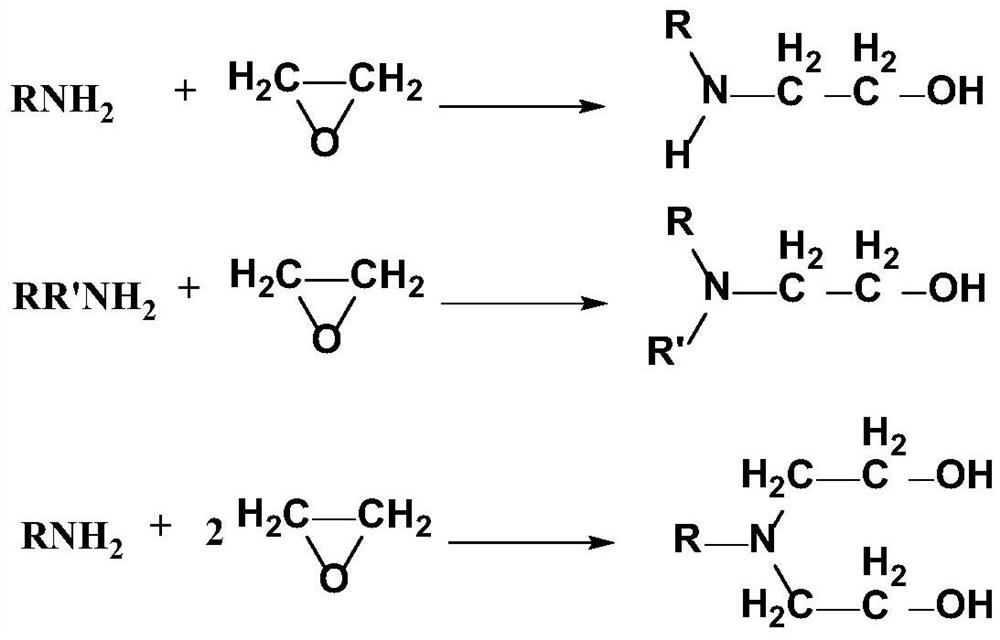
A kind of preparation method of alkanolamine
A technology of alkanolamines and amines, which is applied in the preparation of organic compounds, chemical instruments and methods, preparation of aminohydroxy compounds, etc., to achieve the effects of improving product purity, reducing the generation of colored impurities, and promoting complete progress.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
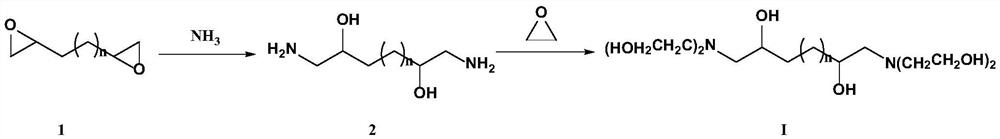
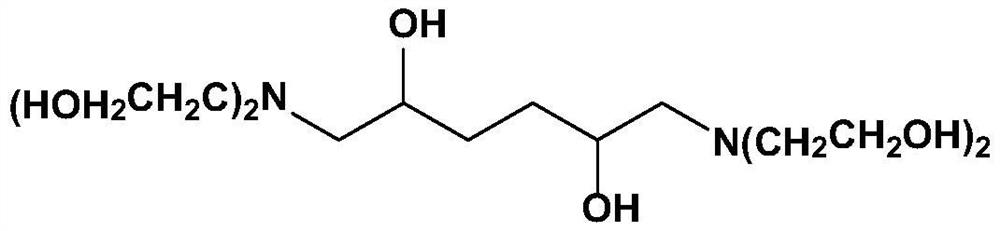
[0042] Example 1: Preparation of 1,6-bis(bis(2-hydroxyethyl)amino)hexane-2,5-ol
[0043]
[0044] Add liquid ammonia 374g, 1,5-hexadiene diepoxide 1141g, catalyst CuI76g, cocatalyst ZrO in the reactor 2 25g and a small amount of hexamethylenetetramine (about 28g), then replace the air in it with nitrogen, seal the reactor and heat it to 60°C, react for 4h; then feed ethylene oxide gas into it, and react The temperature was increased to 85°C, the pressure in the reactor was maintained at 0.25-0.3Mpa, and the reaction was continued for 5h. After the reaction is completed, filter and recover the catalyst while it is hot, and rectify the filtrate to remove low-boiling substances first, then gradually heat to 140-150°C under a vacuum of 10 mmHg to recover the mixture of ethanolamine and diethanolamine, and the remaining bottom liquid is the target product 1 ,6-bis(bis(2-hydroxyethyl)amino)hexan-2,5-ol 3127g, based on 1,5-hexadiene diepoxide, the yield is 96.4%, and the purity ...
Embodiment 2
[0048] Example 2: Preparation of 1,8-bis(bis(2-hydroxyethyl)amino)octan-2,7-ol
[0049]
[0050] Add liquid ammonia 374g, 1,7-octadiene diepoxide 1422g, catalyst CuI76g, cocatalyst ZrO in the reactor 2 25g and a small amount of hexamethylenetetramine (about 28g), then replace the air therein with nitrogen, seal the reactor and heat it to 65°C, and react for 4.5h; then feed ethylene oxide gas into it, and The reaction temperature was increased to 90°C, the pressure in the reactor was maintained at 0.28-0.32Mpa, and the reaction was continued for 6h. After the reaction is completed, filter and recover the catalyst while it is hot, and rectify the filtrate to remove low-boiling substances first, then gradually heat to 140-150°C under a vacuum of 10 mmHg to recover the mixture of ethanolamine and diethanolamine, and the remaining bottom liquid is the target product 1 ,8-bis(bis(2-hydroxyethyl)amino)octan-2,7-ol 3344g, based on 1,7-octadiene diepoxide, the yield is 94.9%, and ...
Embodiment 3
[0054] Example 3: Preparation of 1,6-bis(bis(2-hydroxyethyl)amino)hexane-2,5-ol
[0055] Add liquid ammonia 374g, 1,5-hexadiene diepoxide 1141g, catalyst CuI76g, cocatalyst ZrO in the reactor 2 15g and a small amount of hexamethylenetetramine (about 14g), then replace the air therein with nitrogen, seal the reactor and heat it to 60°C, react for 5h; then feed ethylene oxide gas into it, and react The temperature was increased to 85°C, the pressure in the reactor was maintained at 0.25-0.3Mpa, and the reaction was continued for 5h. After the reaction is completed, filter and recover the catalyst while it is hot, and rectify the filtrate to remove low-boiling substances first, then gradually heat to 140-150°C under a vacuum of 10 mmHg to recover the mixture of ethanolamine and diethanolamine, and the remaining bottom liquid is the target product 1 ,6-bis(bis(2-hydroxyethyl)amino)hexan-2,5-ol 3088g, based on 1,5-hexadiene diepoxide, the yield is 95.2%, the purity is 98.5%; , t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com