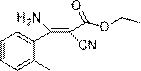
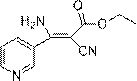
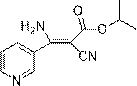
Synthesis method of cyanoacrylate compound
A technology of cyanoacrylate and synthesis method, which is applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry, etc., can solve problems such as production capacity limitation, potential risks to human health and environment, and improve production efficiency. , the effect of eliminating human health hazards and ecological environment pollution, and improving the utilization rate of equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment -1
[0054] step one,
[0055] Reaction formula:
[0056]
[0057] Operation process: the synthesis of ethyl phenyl carbamidate is carried out with reference to the relevant content of the document J.hetercyclic.Chem.33. 1903 (1996). Operation process: at 5-10°C, 150g of hydrogen chloride gas is bubbled into a solution of about 4.0 moles of benzonitrile and 8.0 moles of ethanol in 2000 milliliters of toluene. And stirred at room temperature (25°C) for 20 hours, and the white solid was filtered off. This white solid was dissolved in 3000 milliliters of absolute ethanol and neutralized to a pH value of 7-8.5 with triethylamine, the solvent was evaporated to dryness under reduced pressure, triethylamine hydrochloride was filtered out, and the filter cake was washed 3 times with 200ml dichloromethane, and combined Dichloromethane and part of the ethanol were removed from the filtrate and washing liquid under reduced pressure, and the remaining oil was distilled under reduced press...
Embodiment 2
[0061] step one,
[0062] Reaction formula:
[0063]
[0064] Operation process: Add 121g (1.0mol) of benzamide, 169.4g (1.10mol) of diethyl sulfate, and 860g of toluene into a four-necked flask equipped with mechanical stirring, a thermometer, and a reflux condenser. Raise the temperature to 110-120°C for reflux reaction for 4 hours, then cool down to 25°C. Neutralize to pH 7.0-8.5 with 5% sodium hydroxide solution, separate the phases, and dry the organic layer with 25 g of anhydrous sodium sulfate. After removing toluene, vacuum distillation cut off the fraction at 104-105°C at 2 mmHg to obtain 130 g of ethyl benzimidate with a purity of 98% by GC analysis.
[0065] step two
[0066] Reaction formula:
[0067]
[0068] Operation process: Add 149g (1.0mol) of ethyl phenylcarboximidate, 113g of ethyl cyanoacetate (1.0mol), DMAP (4-di methylaminopyridine) 0.1g to 80°C, and reacted at this temperature for 2 hours, then raised to 100°C, and kept at this temperature fo...
Embodiment 3
[0070] step one
[0071] Reaction formula:
[0072]
[0073] Operation process: the synthesis of ethyl phenyl carbamidate is carried out with reference to the relevant content of the document J.hetercyclic.Chem.33. 1903 (1996). At 5-10° C., 150 g of hydrogen chloride gas is bubbled into a solution of about 4.0 moles of benzonitrile and 8.0 moles of ethanol in 2000 milliliters of toluene. And stirred at room temperature (25° C.) for 20 hours to obtain a white solid which was filtered out. Add 10% sodium carbonate solution to neutralize to a pH value of 7.0-8.5, separate phases, wash the organic layer with (1%) sodium bicarbonate three times, dry with 50g of anhydrous sodium sulfate, filter out sodium sulfate crystals, and store at 80°C, Toluene was removed under a vacuum of 0.98 to obtain 586 g of ethyl benzoate, (content 93%, quantitative analysis by gas chromatography)
[0074] step two
[0075]
[0076] Operation process: In a four-necked glass bottle equipped with...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com