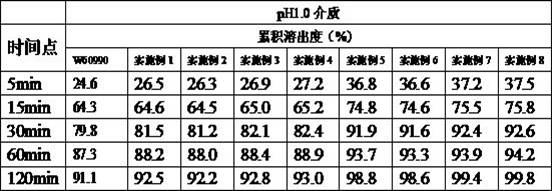
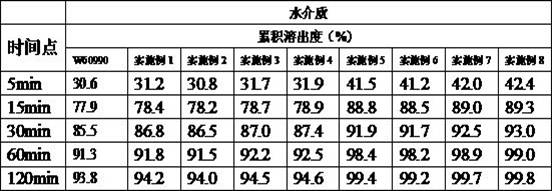
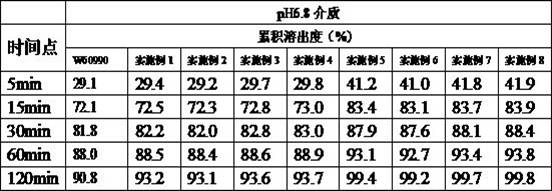
Celecoxib capsule and preparation method thereof
A technology of celecoxib and capsules, which is applied in the field of celecoxib capsules and its preparation, can solve the problems of poor dissolution effect, large specific surface area, and small weight difference, and achieve small weight difference, large specific surface area, and release The effect of drug stabilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0055] The present invention also includes the preparation method of celecoxib capsules, comprising the following steps:
[0056] In parts by weight, add 25-80 parts of celecoxib, 19-72 parts of blank pellet core and 0.2-1 part of absorption enhancer into the centrifugal fluidized bed, turn on the blower, and set the blowing airflow to 2.0-2.5m3 / min , the inlet air temperature is 45~55℃, and the rotating speed of the turntable is 180~220r / min;
[0057] The absorption enhancer is mannitol or / and sorbitol;
[0058] Add 0.5~2 parts of wetting agent into purified water to prepare an aqueous solution with a mass concentration of 12~18%;
[0059] Turn on the peristaltic pump, control the pump speed at 14~18r / min, the nozzle diameter of the spray gun is 0.8mm, and the atomization pressure is 0.04~0.06MPa, spray the above aqueous solution into the centrifugal fluidized bed, granulate and dry, and fill to In the capsule shell, celecoxib capsules are obtained;
[0060] The wettin...
Embodiment 1
[0075] A celecoxib capsule, comprising the following materials: celecoxib 250g, blank core 190g, wetting agent 5g and absorption accelerator 2g;
[0076] Described wetting agent is sodium lauryl sulfate;
[0077] The absorption enhancer is mannitol.
Embodiment 2
[0079] A celecoxib capsule, comprising the following materials: celecoxib 800g, blank core 720g, wetting agent 20g and absorption accelerator 10g;
[0080] The wetting agent is sodium lauroyl acid;
[0081] The absorption enhancer is sorbitol.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com