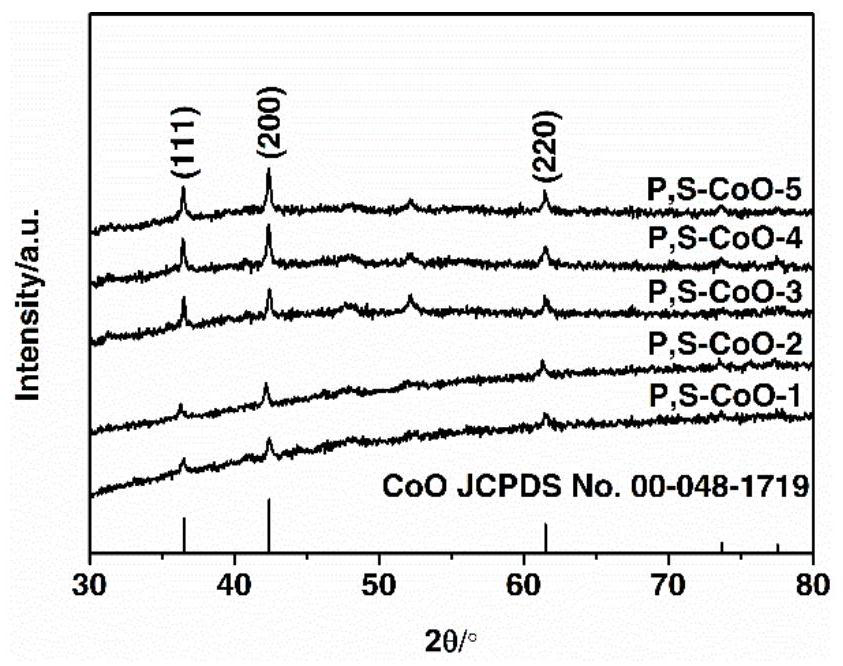
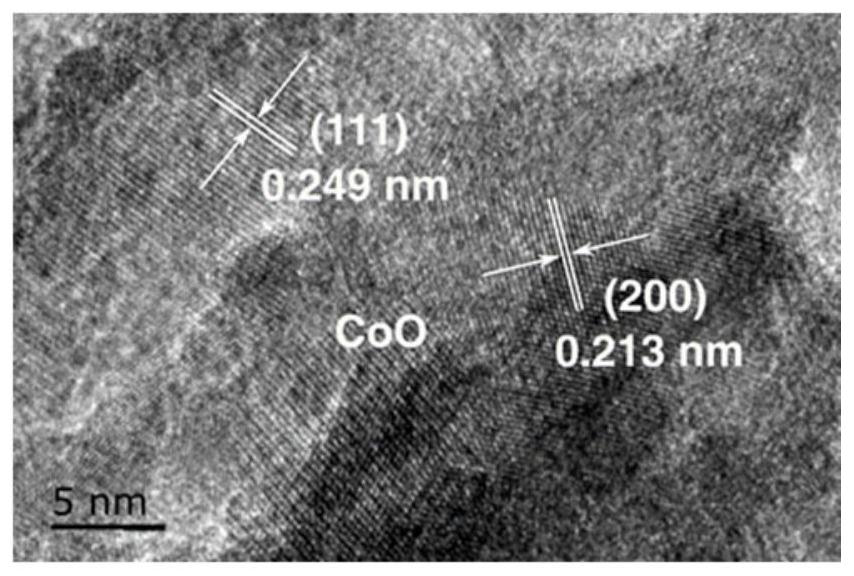
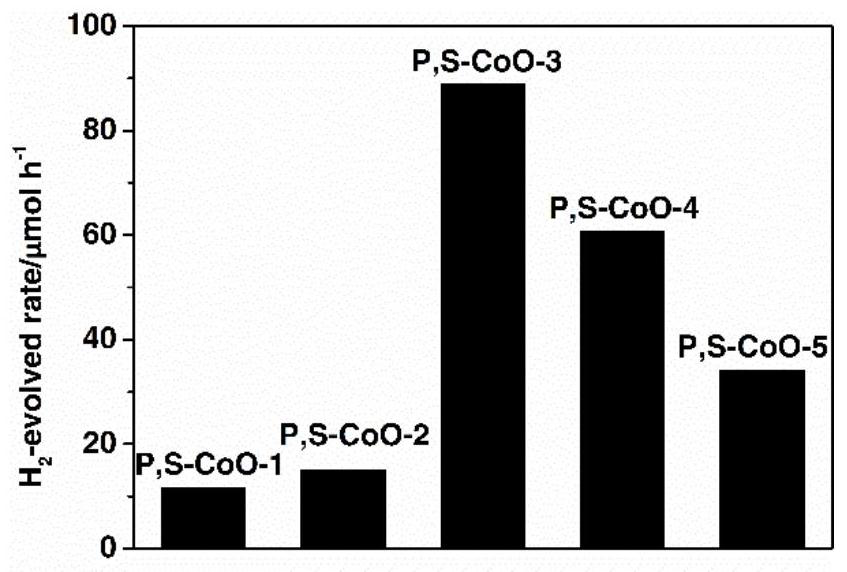
Preparation method of phosphorus and sulfur co-modified cobaltous oxide and application of phosphorus and sulfur co-modified cobaltous oxide in photocatalytic decomposition of water
A cobalt oxide and co-modification technology, applied in chemical instruments and methods, catalyst activation/preparation, physical/chemical process catalysts, etc., can solve problems such as poor stability of photocatalytic water splitting, and achieve improved hydrogen production efficiency and good visible light. The effect of catalytic hydrogen production activity and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The preparation method of phosphorus and sulfur co-modified cobaltous oxide comprises the following steps:
[0030] Step 1: Weigh 1.6mol NaOH (or KOH) and 1mmol Co(NO 3 ) 2 ·6H 2 O (or cobalt chloride) was added to 20mL deionized water respectively, stirred and dissolved to obtain NaOH aqueous solution and Co(NO 3 ) 2 aqueous solution. Then Co(NO 3 ) 2 The aqueous solution was added dropwise to aqueous NaOH and stirred at 25 °C for 30 min. Subsequently, the resulting mixed solution was transferred to a 100 mL polytetrafluoroethylene liner and sealed with a stainless steel reactor, and reacted at 100 ° C for 24 h. After cooling to room temperature naturally, the reactant was centrifuged and washed five times with deionized water and placed in a vacuum. Dry in an oven at 50°C for 12 hours to finally obtain Co(OH) 2 .
[0031] Step 2: Choose NaH 2 PO 2 ·H 2 O and sulfur powder are used as phosphorus source and sulfur source respectively. Phosphorus and sulfur c...
Embodiment 1
[0036] Step 1: Weigh 160mmol NaOH and 1mmol Co(NO 3 ) 2 ·6H 2 O was added to 20 mL of deionized water, stirred and dissolved to obtain two aqueous solutions. Then Co(NO 3 ) 2 The aqueous solution was added dropwise to NaOH aqueous solution, and stirred at room temperature for 30 min. Subsequently, the resulting mixed solution was transferred to a 100 mL polytetrafluoroethylene liner and sealed with a stainless steel reactor, and reacted at 100 ° C for 24 h. After cooling to room temperature naturally, the reactant was centrifuged and washed five times with deionized water and placed in a vacuum. Dry in an oven at 50°C for 12 hours, and the final sample is Co(OH) 2 .
[0037] Step 2: 1mmol Co(OH) obtained in Step 1 2 with 5mmol NaH 2 PO 2 ·H 2 O and 1 mmol of sulfur powder were mixed and ground evenly, and the ground powder was calcined at 300 °C for 2 h under Ar atmosphere (heating rate 2 °C / min), and after cooling in the furnace, the obtained sample was washed 4 tim...
Embodiment 2
[0043] Step 1: Weigh 160mmol NaOH and 1mmol Co(NO 3 ) 2 ·6H 2 O was added to 20 mL of deionized water, stirred and dissolved to obtain two aqueous solutions. Then Co(NO 3 ) 2 The aqueous solution was added dropwise to NaOH aqueous solution, and stirred at room temperature for 30 min. Subsequently, the resulting mixed solution was transferred to a 100 mL polytetrafluoroethylene liner and sealed with a stainless steel reactor, and reacted at 100 ° C for 24 h. After cooling to room temperature naturally, the reactant was centrifuged and washed five times with deionized water and placed in a vacuum. Dry in an oven at 50°C for 12 hours, and the final sample is Co(OH) 2 .
[0044] Step 2: 1mmol Co(OH) obtained in Step 1 2 with 5mmol NaH 2 PO 2 ·H 2 O and 2mmol sulfur powder were mixed and ground evenly, and the ground powder was calcined at 300°C for 2h under Ar atmosphere (heating rate 2°C / min), and after cooling with the furnace, the obtained sample was washed 4 times wi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com