Method for realizing purification of recombinant protein by using antifreeze protein-intein as purification tag and application
An antifreeze protein and purification tag technology, applied in the field of separation and purification of exogenous recombinant proteins, can solve the problems of increased cost of protease, separation steps, complicated process, difficulty in scale, etc., and achieve easy molecular cloning operation, simple operation and labor saving. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 Construction of pET-24a-GST-Mxe GyrA-linker-AFP expression vector
[0048] (1) Synthesize the following primers respectively:
[0049] SEQ ID NO.4: Primer P1:
[0050] 5'-GGAATTC CATATG ATGTCCCCTATACTAGGTTATTG-3', where the underlined part is QuickCut TM Nde I recognition site.
[0051] SEQ ID NO.5: Primer P2:
[0052] 5'TGCATCTCCCGTGATGCA CATTCGCAT TTTTGGAGGATGGTCGCCACCACCAAAC-3', wherein the underlined part is the base sequence corresponding to the three amino acid MRMs.
[0053] SEQ ID NO.6: Primer P3:
[0054] 5'-GTTTGGTGGTGGCGACCATCCTCCAAAAA ATGCGAATG TGCATCACGGGAGATGCA-3', wherein the underlined part is the base sequence corresponding to the three amino acid MRMs.
[0055] SEQ ID NO.7: Primer P4:
[0056] 5'-ATCACTC AAGCTT AGCGTGGCTGACGAACCCGTTC-3', where the underlined part is QuickCut TM Hind III recognition site.
[0057] (2) Under the guidance of the above-mentioned P1 and P2 primers, the GST gene was amplified by PCR using the pGEX-4...
Embodiment 2
[0059] Example 2 The heterologous expression of GST-Mxe GyrA-linker-AFP
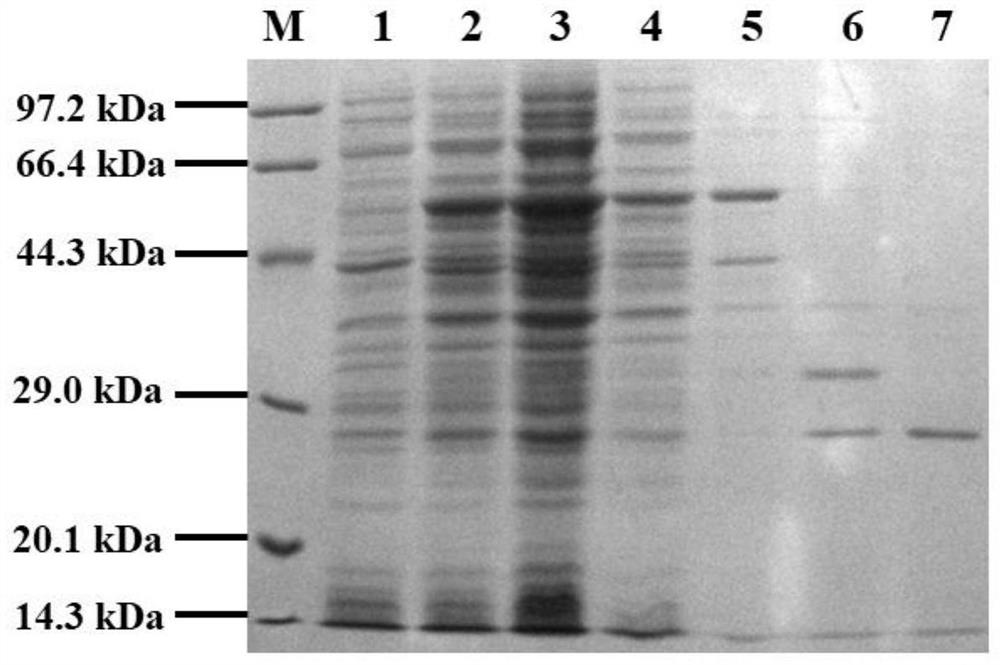
[0060] The recombinant expression plasmid pET24a-GST-Mxe GyrA-linker-AFP obtained in Example 1 was transferred into Escherichia coli BL21 (DE3) competent cells by the heat shock method, and a single colony was picked in a cell containing 50 μg / mL kanamycin Incubate in 5 mL of LB medium at 37°C for 12 hours, then transfer to TB medium containing 50 μg / mL kanamycin at a ratio of 2% (v / v), and continue culturing at 37°C until OD 600 When the concentration is 0.6-0.8, add IPTG to a final concentration of 0.5mM. After induction at 16°C for 20 h, the bacterial cells were collected and resuspended in 50 mM Tris-HCl buffer at pH 8.5 for ultrasonication and centrifugation, and the supernatant was collected for SDS-PAGE electrophoresis.
Embodiment 3
[0061] Example 3 Separation and purification of GST using antifreeze protein-intein tag
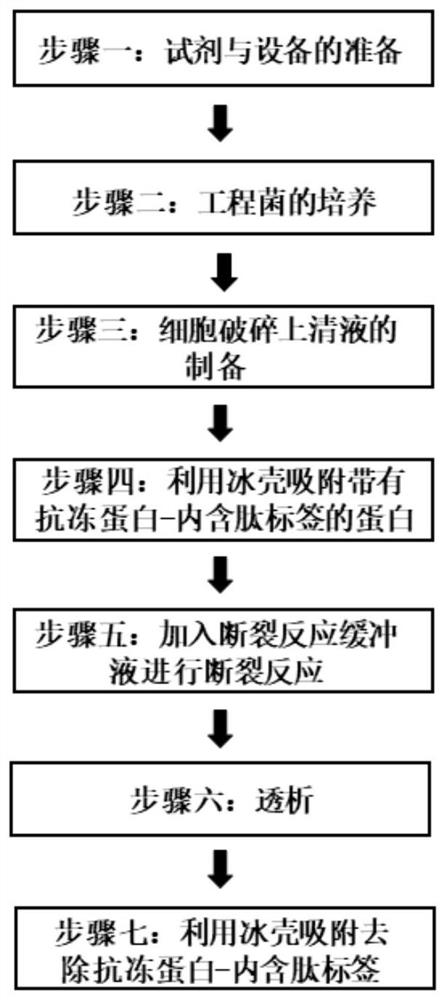
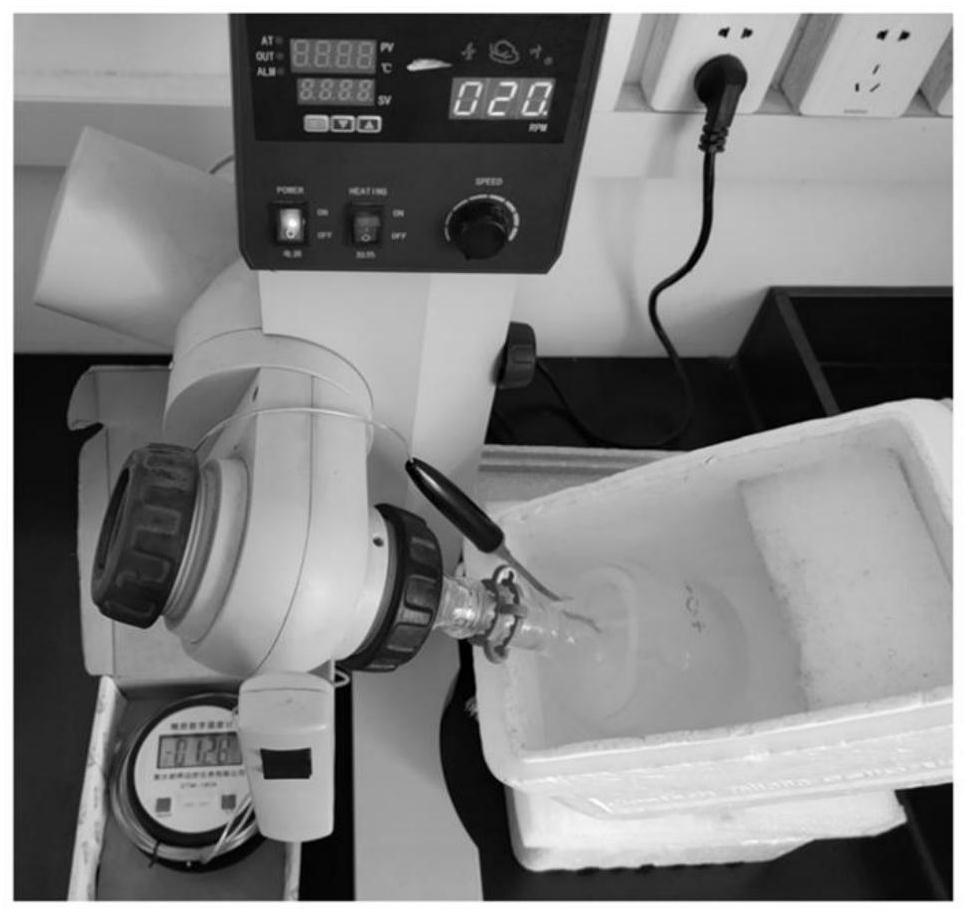
[0062] Prepare the ice shell: Measure 100mL of pre-cooled deionized water into a 500mL round bottom flask, immerse the round bottom flask in the freezing liquid and rotate rapidly for 1min so that part of the deionized water in the round bottom flask forms a close fit to the round bottom flask For the ice shell on the inner wall, pour out the remaining deionized water, continue to immerse the round-bottomed flask with the ice shell in the ice-adsorption freezing liquid and rotate for 2 minutes, until the ice shell has evenly distributed cracks, and then follow-up experiments can be carried out.
[0063] Dilute the supernatant of broken cells to 1 mg / mL, pre-cool with ice-water mixture, and slowly drop on the surface of the ice shell in the round-bottom flask, such as figure 2 As shown, connect the round-bottomed flask to the rotary evaporator and immerse the round-bottomed flask in the i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com