Continuous synthesis method of benzophenone compound
A technology of benzophenones and chemical synthesis, which is applied in the direction of preparation of organic compounds, condensation preparation of carbonyl compounds, chemical instruments and methods, etc., can solve the problems of short reaction time and good environmental protection, and achieve small footprint and high conversion The effect of high efficiency and low human resources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
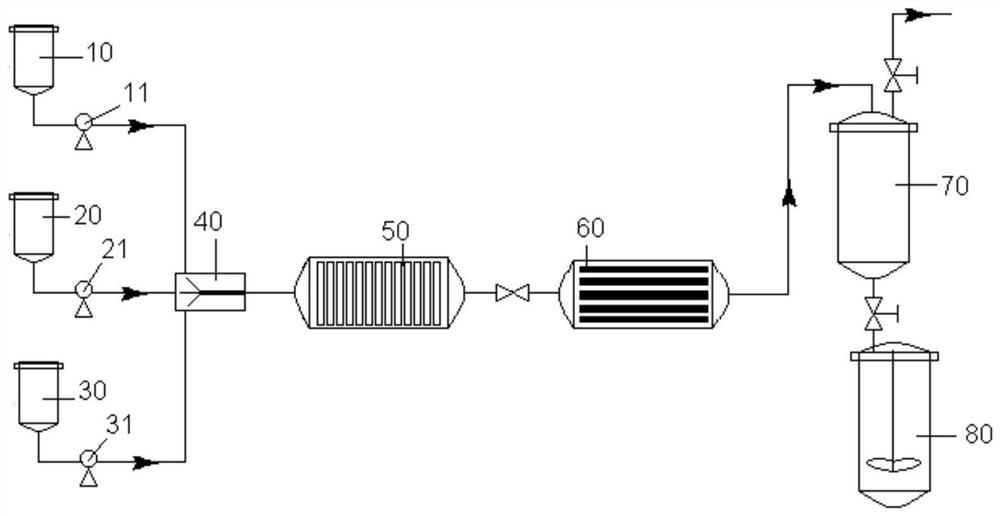
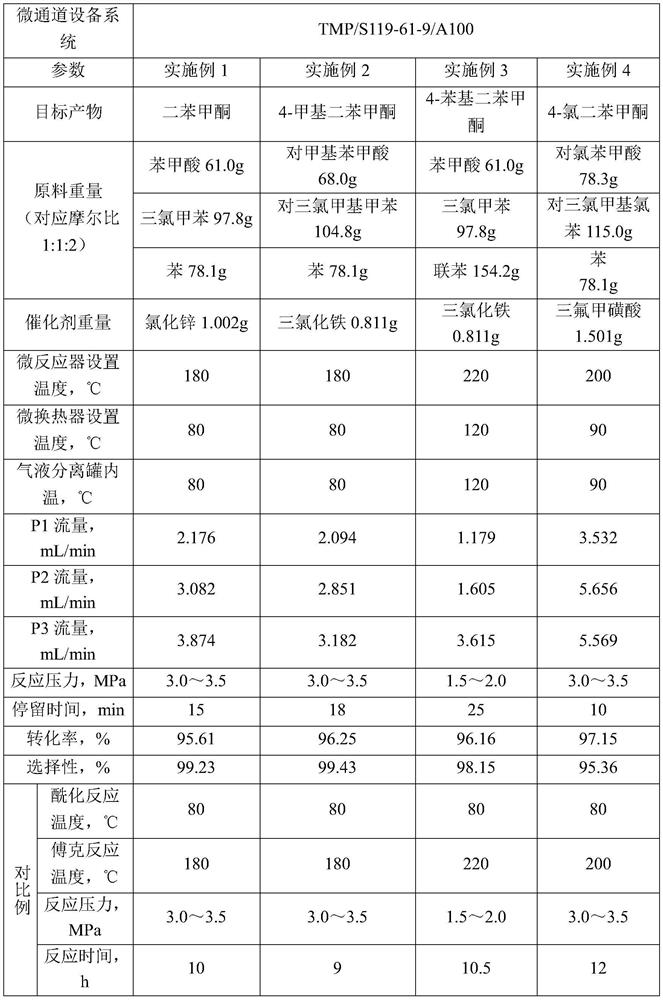
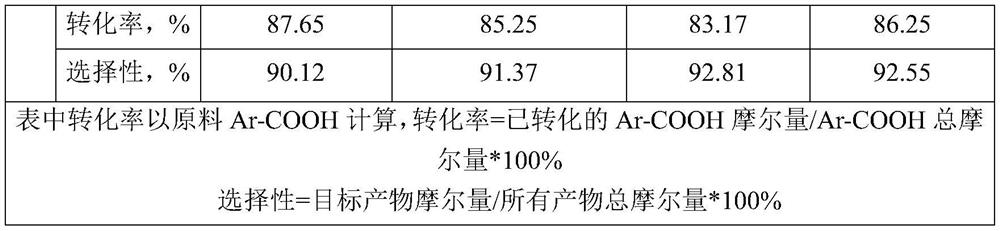
[0037] Example uses figure 1 The shown device prepares benzophenone compounds. The microchannel equipment system used is provided by Shanghai Timo Fluid Technology Co., Ltd., the model is TMP / S119-61-9 / A100, and the channel inner diameter of the microchannel reactor is 4000 μm. Synthetic methods include:
[0038] The substituted or unsubstituted benzoic acid is stored in the first raw material storage tank 10 as the first organic matter, the substituted or unsubstituted trichlorotoluene is stored in the second raw material storage tank 20 as the second organic matter, and the benzene or biphenyl Stored in the third raw material storage tank 30 as the third organic matter. Through the first feed pump 11 (P1), the second feed pump 21 (P2), the third feed pump 31 (P3), the first organic matter, the second organic matter and the third organic matter are input into the micro-mixer 40 respectively Mix to obtain a mixed solution. Then the above-mentioned mixed solution is sent to ...
Embodiment 5
[0044] The difference from Example 1 is that the molar ratio of the first organic compound, the second organic compound and the third organic compound is 1:0.95:2.1.
[0045] The conversion rate of the product is 90.71%, and the selectivity is 99.27%.
Embodiment 6
[0047] The difference from Example 1 is that the molar ratio of the first organic compound, the second organic compound and the third organic compound is 1:1.05:1.9.
[0048] The conversion rate of the product is 91.87%, and the selectivity is 98.26%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com