Immobilized metal ion affinity chromatography filler, chromatographic column and preparation method thereof
A technology for immobilizing metals and chromatographic columns, applied in the field of chromatography, can solve the problems of low selectivity, poor chelating performance, high price, etc., and achieve good physical and chemical stability, high repeatability and yield, and low preparation cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
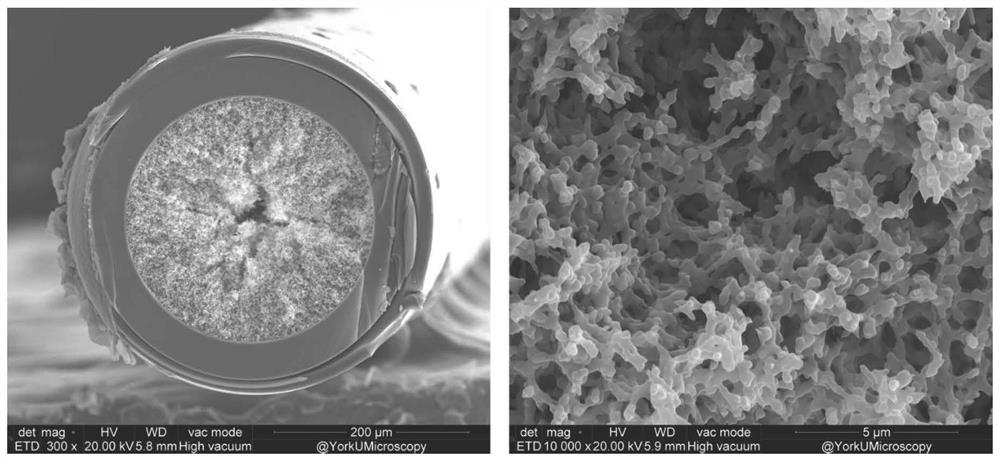
[0037] Example 1 Preparation of ATP-modified immobilized metal ion affinity capillary monolithic column
[0038] The ATP-modified immobilized metal ion affinity capillary monolithic column prepared by a one-step reaction method in the present invention specifically comprises the following steps:
[0039] S1. Add 740 microliters (about 1000 mg) of potassium water glass (modulus 3.3, Baume degree 40) to the micro-reaction flask, and slowly add 6 microliters (about 6 mg) of γ-glycidyl ether oxypropylene under stirring at room temperature Trimethoxysilane (GLYMO, Cas number: 2530-83-8), and continued to stir well at room temperature for 30 minutes.
[0040] S2. Weigh 7.5 mg of adenosine triphosphate disodium, dissolve it in 160 microliters of deionized water, slowly add it to the reaction solution in step S1, and continue to stir fully at room temperature for 30 minutes.
[0041] S3. Take 60 microliters (about 68 mg) of formamide, mix it with 40 microliters of deionized water, sl...
Embodiment 2
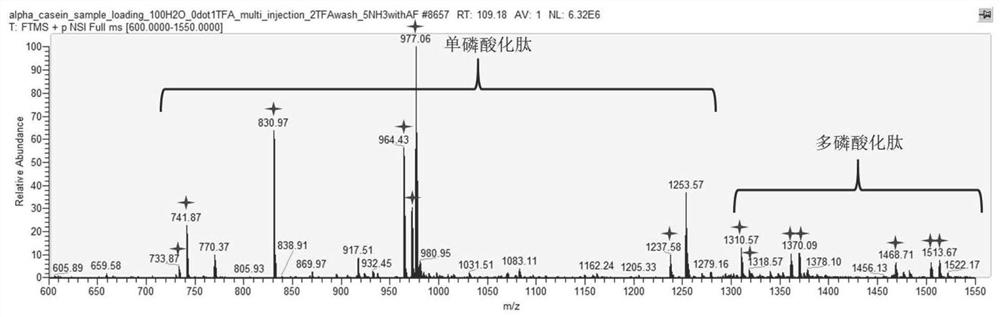
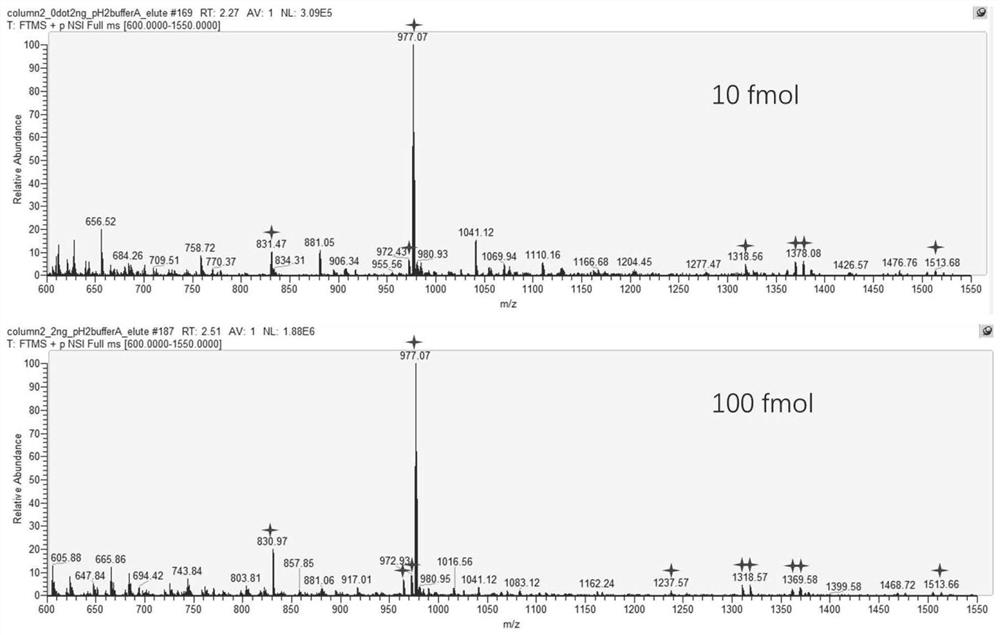
[0046] Example 2 The ability of the ATP-modified immobilized metal ion affinity capillary monolithic column prepared in Example 1 of the present invention to enrich phosphorylated peptides was comprehensively investigated.
[0047] The ATP-modified immobilized metal ion affinity capillary monolithic column prepared in Example 1 of the present invention was used for the enrichment of phosphorylated peptides obtained by treating standard phosphorylated protein α-casein or BSA with trypsin. The enrichment performance was examined from the aspects of site coverage, detection limit and selectivity.
[0048]2mg / ml BSA dissolved in 50mM ammonium bicarbonate solution was added to DTT to 10mM and heated at 56°C for 30min, then added iodoacetamide to 40mM and incubated in the dark for 40min to break the disulfide bond. Take 1 mg of standard phosphorylated protein α-casein and 1 mg of BSA that breaks disulfide bonds, dissolve in 1 ml of 50 mM ammonium bicarbonate, and treat with 20 μg tr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com