High-valence metal ion doped oxygen vacancy-rich cobalt oxide nano composite material and preparation and application thereof
A technology of nanocomposite materials and metal ions, which is applied in the field of cobalt oxide nanocomposites doped with high-valence metal ions and rich in oxygen vacancies and its preparation, to achieve good reproducibility, increase diversity, and improve intrinsic conductivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
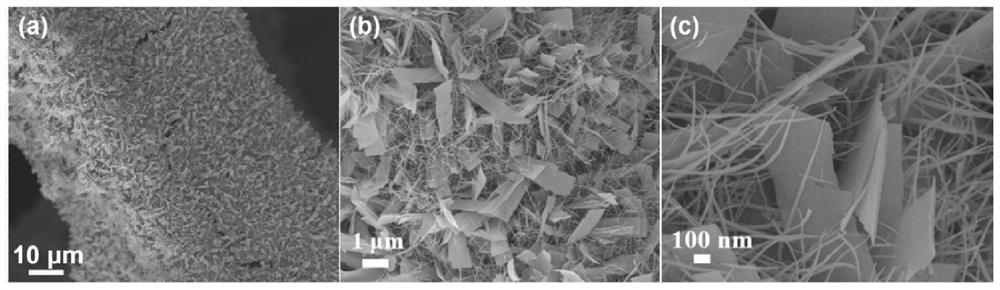
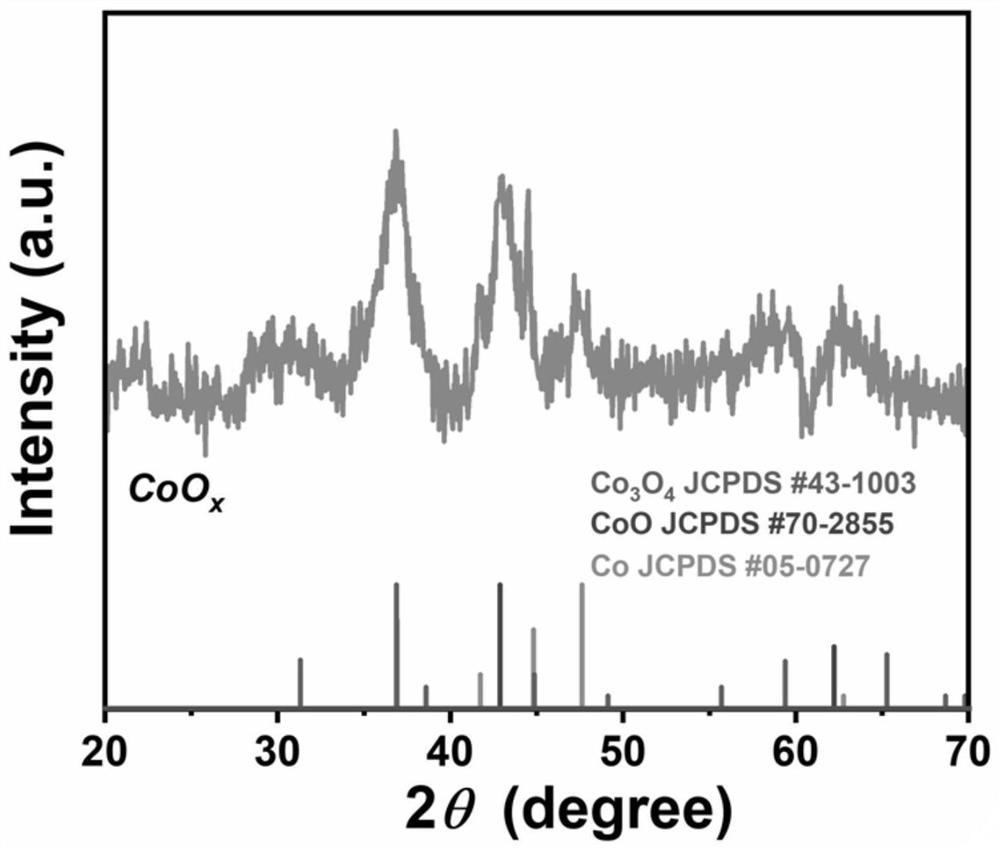
[0042] Example 1: CoO x Preparation and its oxygen evolution performance (without doping high-valence metal ions)
[0043] Take cobalt nitrate (0.582g, 2mmol) and dissolve it in 20mL DI, take urea (0.6g, 10mmol) and dissolve it in 20mL DI, mix the two evenly, transfer the solution to a 50mL hydrothermal kettle; add pretreated 4×1cm Foam nickel substrate, react at 120°C for 6 hours. After the reaction was completed, it was naturally cooled to room temperature, and the nickel foam substrate was taken out, rinsed several times with deionized water and absolute ethanol, and vacuum-dried at 60°C for 12 hours to obtain the precursor material.
[0044] The prepared nickel foam (loaded with precursor material 0.01g) was placed in a porcelain boat, and 1.0g NaBH was weighed 4 Placed in another porcelain boat. loaded with NaBH 4 The porcelain boat is placed upstream, and the porcelain boat filled with nickel foam is placed in the middle section of the tube furnace. Under nitrogen a...
Embodiment 2
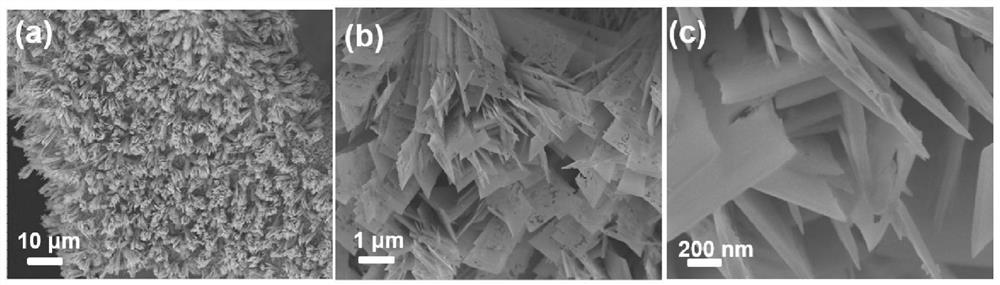
[0047] Example 2: W / CoO x Preparation of -1 and its oxygen evolution performance
[0048] Dissolve cobalt chloride (0.582g, 2.45mmol) and sodium tungstate (0.04g, 0.12mmol) in 20mL DI, and dissolve urea (0.6 g, 10mmol) in 20mL DI. 50mL hydrothermal kettle; add pretreated 4×1 cm foam nickel substrate, and react at 120°C for 6 hours. After the reaction was completed, it was naturally cooled to room temperature, and the nickel foam substrate was taken out, rinsed several times with deionized water and absolute ethanol, and vacuum-dried at 60°C for 12 hours to obtain the precursor material.
[0049] The prepared nickel foam (loaded with 0.007g of precursor material) was placed in a porcelain boat, and 1.0g of NaBH was weighed 4 Placed in another porcelain boat. loaded with NaBH 4 The porcelain boat is placed upstream, and the porcelain boat filled with nickel foam is placed in the middle section of the tube furnace. In a nitrogen atmosphere, the temperature was raised to 350°...
Embodiment 3
[0051] Example 3: Mo / CoO x Preparation of -2 and its oxygen evolution performance
[0052] Dissolve cobalt acetate (0.582g, 2.3mmol) and sodium molybdate (0.12g, 0.5mmol) in 20mL DI, dissolve urea (0.6g, 10mmol) in 20mL DI, mix the two evenly, and transfer the solution to 50mL In a hydrothermal kettle; add a pretreated 4×1cm foam nickel substrate, and react at 120°C for 6 hours. After the reaction was completed, it was naturally cooled to room temperature, and the nickel foam substrate was taken out, rinsed several times with deionized water and absolute ethanol, and vacuum-dried at 60°C for 12 hours to obtain the precursor material.
[0053] The prepared nickel foam (loaded with precursor material 0.008g) was placed in a porcelain boat, and 1.0g NaBH was weighed 4 Placed in another porcelain boat. loaded with NaBH 4 The porcelain boat is placed upstream, and the porcelain boat filled with nickel foam is placed in the middle section of the tube furnace. In a nitrogen atmo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com