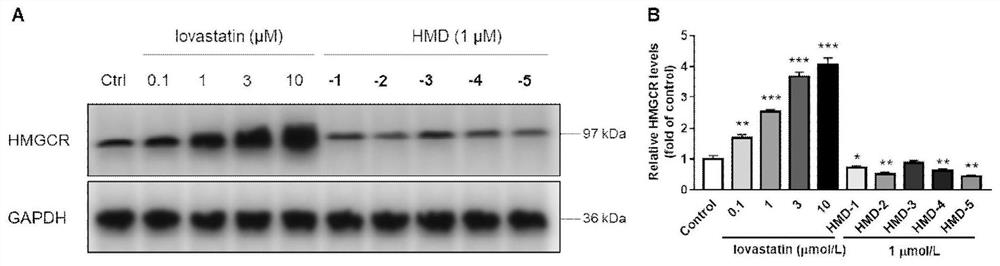
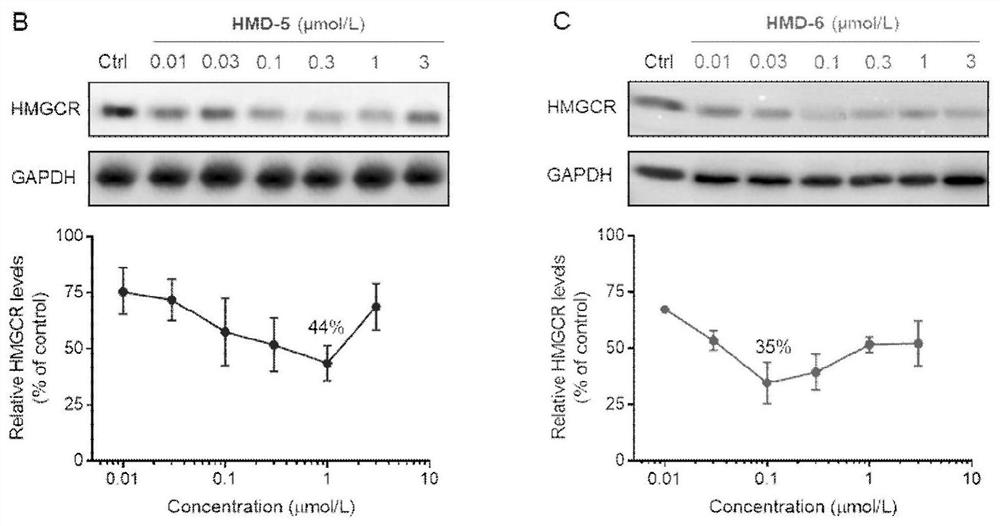
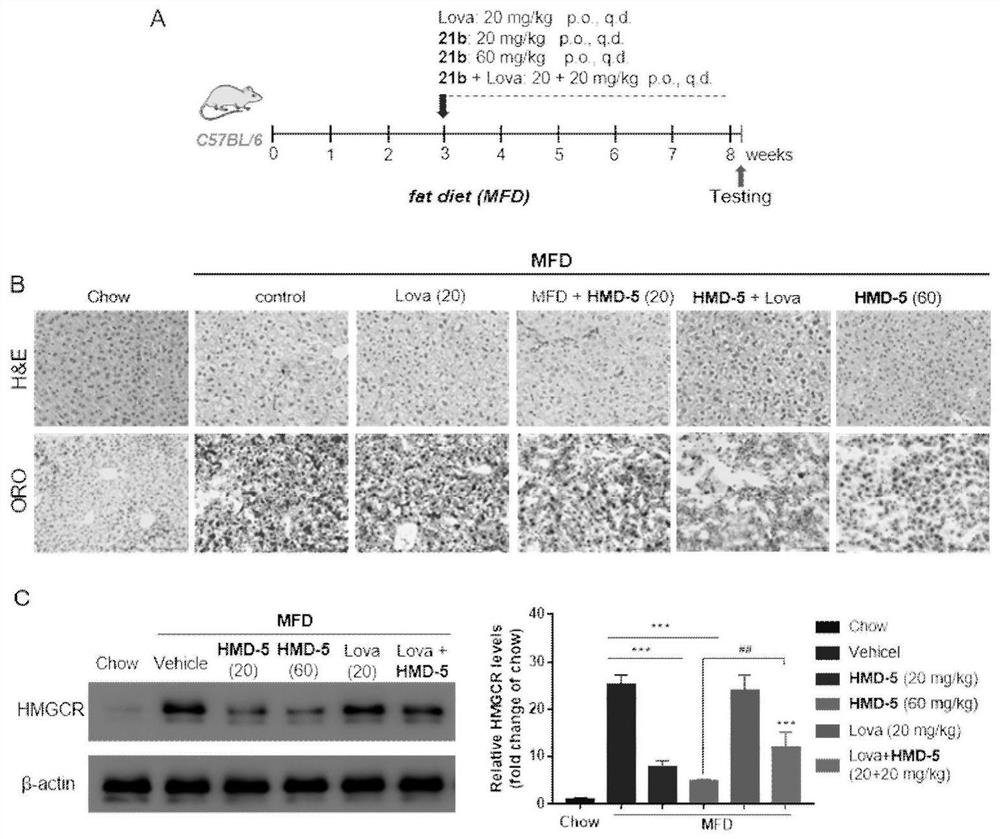
Compound or medicinal salt thereof for targeted ubiquitination degradation of HMGCR, preparation method and application
A compound and ubiquitination technology, applied in the field of medicine, can solve the problems of unsatisfactory reduction of LDL-C, and achieve the effect of significant degradation activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: Synthesis of CRBN ligand derivatives. Compound 1 (0.28g, 1.0mmol) was dissolved in anhydrous DMF, and then amine compounds 2a-2c (1.0mmol) of different lengths, DIPEA (0.4g, 3.0mmol), DIPEA (0.4g, 3.0mmol), 90 °C overnight. After the completion of the reaction as detected by TLC, the reaction solution was poured into water, extracted with dichloromethane, the organic layer was washed with saturated brine, dried over anhydrous sodium sulfate, concentrated, and column chromatographed to obtain yellow solids 3a-3c.
[0036] The synthetic route is as follows:
[0037]
[0038] Reagents and conditions: (i) N, N-Diisopropylethylamine, DMF, 90℃, 12h
[0039] (1) Synthesis of N-2-(2,6-dioxopiperidin-3-yl)-4-propylcarbamate tert-butyl isoindoline-1,3-dione (3a)
[0040]
[0041] Yellow solid (0.22g, 45% yield). 1H NMR (300MHz, CDCl 3 )δ8.31(s, 1H), 7.53(m, 1H), 7.13(d, J=7.1Hz, 1H), 6.98(d, J=8.6Hz, 1H), 6.49(t, J=5.6Hz, 1H), 5.02(s, 1H), 4.95(dd, J=11.8, ...
Embodiment 2
[0048] Example 2: Synthesis of VHL ligand derivatives. Compound 4 (0.4g, 0.93mmol) was dissolved in anhydrous DMF, and then under nitrogen protection, alkyl acid compounds 5a-2b (0.93mmol) of different lengths, DIPEA (0.3g, 1.86mmol) and HATU (0.35g, 0.93mmol), react overnight at room temperature. After the completion of the reaction as detected by TLC, the reaction solution was poured into water, extracted with ethyl acetate, the organic layer was washed with saturated brine, dried over anhydrous sodium sulfate, concentrated, and column chromatographed to obtain yellow solids 6a-6b.
[0049] The synthetic route is as follows:
[0050]
[0051] Reagents and conditions: (i) N, N-Diisopropylethylamine, HATU, DMF, rt, 12h
[0052] (1) tert-butyl (8-(((S)-1-((2S,4R)-4-hydroxyl-2-((4-(4-methylthiazol-5-yl)benzyl)carbamoyl )pyrrolidin-1-yl)-3,3-dimethyl-1-oxobut-2-yl)amino)-8-oxooctyl)formamide (6a) synthesis
[0053]
[0054] Gray solid (0.22g, 35% yield). 1 H NMR (300MH...
Embodiment 3
[0058] Example 3: (4R, 6R)-4-((tert-butyldimethylsilyl chloride)oxy)-6-(2-((1S, 2S, 6R, 8S, 8αR)-8-hydroxyl-2 , 6-dimethyl-1,2,6,7,8,8α-hexahydronaphthalene-1-yl) ethyl) tetrahydro-2H-2-pyrone (8), the route is as follows:
[0059]
[0060] Reagents and conditions: (i) kOH, H 2 O-MeOH, reflux, 12h; (ii) 6M HCl, rt, 6h; (iii) TBSCl, imidazole, CH 2 Cl 2 , rt, 6h; (iv) p-nitrophenyl chloroformate, DMAP, pyridine, rt, 16h;
[0061] Lovastatin (18g, 44.6mmol) was added in the mixed solution of water and methanol (H 2 O / MeOH, 1:5, 132mL), KOH (25.2g, 449mmol) was added, refluxed for 12h, methanol was distilled off under reduced pressure, and the mixture was added H 2 O (500mL), CH 2 Cl 2 (100mL) and 6M HCl, adjust pH=2. React at room temperature for 6h, add saturated NaHCO 3 Neutralization. Extraction with DCM, spin-dried to obtain an oil. Dissolved in DCM (85mL), added TBSCl (4.3g, 30mmol) and imidazole (3.4g, 49.6mmol) to it, reacted at room temperature for 6h. Purifie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com