D-A-pi-A-D type fluorescent compound and preparation method thereof
A fluorescent compound, -A-D technology, applied in chemical instruments and methods, photovoltaic power generation, luminescent materials, etc., can solve problems affecting molecular geometry, stacking and electron density distribution, poor molecular planarity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A D-A-π-A-D type fluorescent compound, the synthetic route is:
[0033]
[0034] The preparation process is as follows:
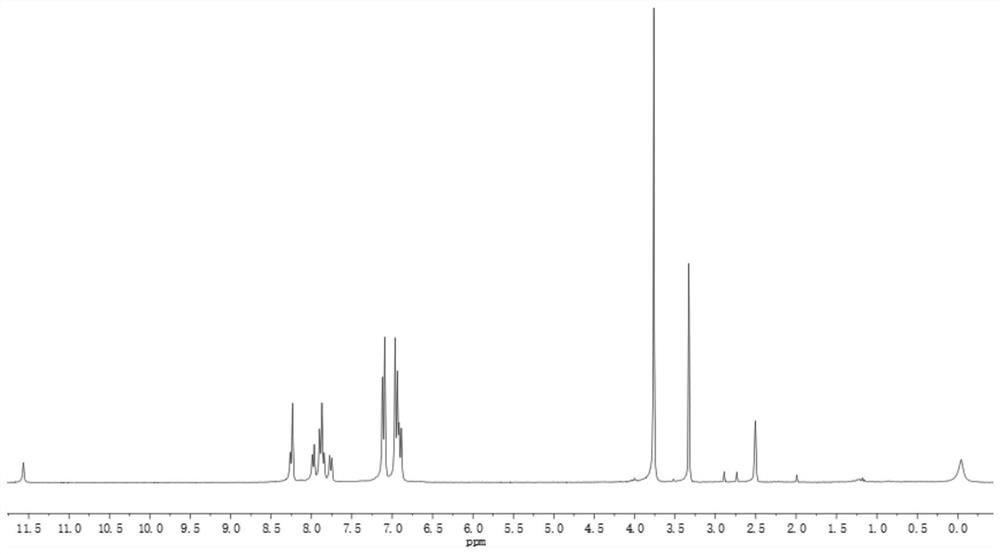
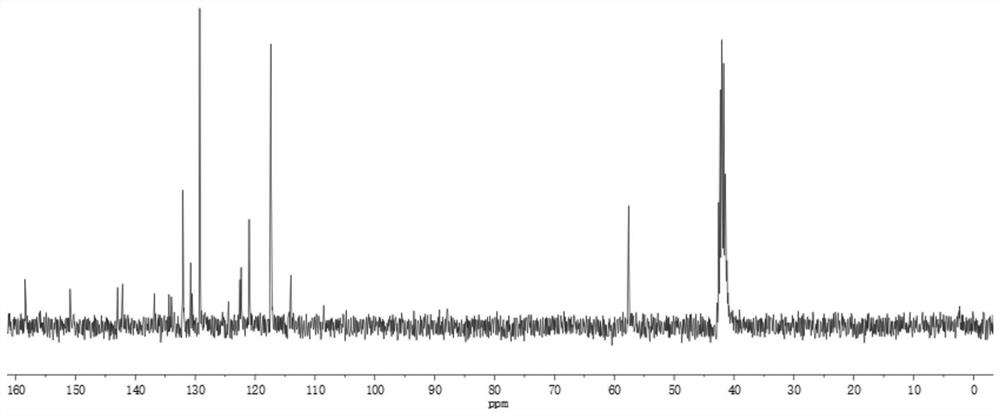
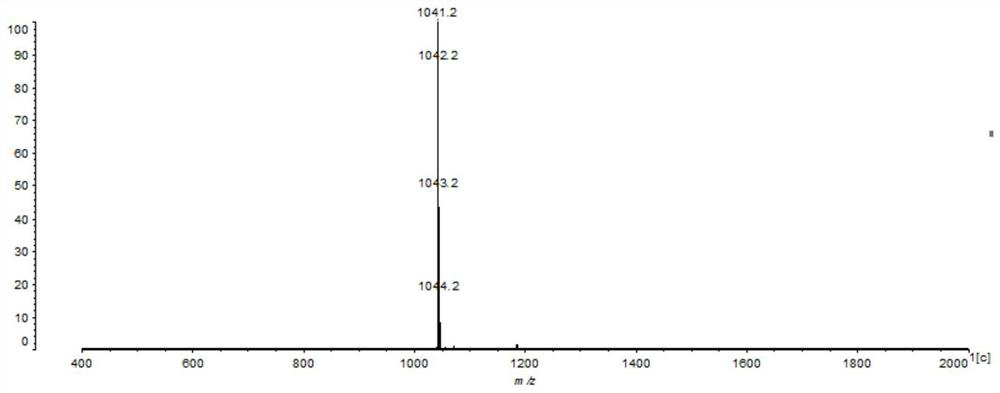
[0035] In the first step, 0.43g compound (I), 0.59g compound (II), 0.06g tetrakis(triphenylphosphine)palladium, 26mL2.0mol / L potassium carbonate aqueous solution and 70mL THF were added to a three-necked flask, Heat under reflux (73°C) for 24h, cool, extract with ethyl acetate and water, collect the organic phase, dry over anhydrous magnesium sulfate, separate and purify by column chromatography (eluent, petroleum ether: ethyl acetate 20:1 (volume ratio )) and dried in a vacuum oven after rotary evaporation to obtain 0.26 g of compound (Ⅲ) as a red powder. Characterization data: 1 H NMR (500MHz, DMSO) δ8.07(d, J=7.7Hz, 1H), 7.84(d, J=8.7Hz, 2H), 7.70(d, J=7.7Hz, 1H), 7.13(d, J =8.8Hz, 4H), 6.98(d, J=8.9Hz, 4H), 6.90(d, J=8.7Hz, 2H), 3.79(s, 6H); 13 C NMR (75MHz, DMSO) δ 158.83, 155.90, 155.18, 151.55, 142.32, 135.41, 135.30, 132.54, 129.88, 12...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal decomposition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com